High risk of hepatic complications in kidney transplantation with chronic hepatitis C virus infection
- PMID: 40784973
- PMCID: PMC12336315
- DOI: 10.1038/s41598-025-15169-4
High risk of hepatic complications in kidney transplantation with chronic hepatitis C virus infection
Abstract
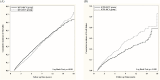
Data on liver issues including liver cirrhosis, hepatocellular carcinoma, and hepatic failure in renal transplant patients with HCV infection are scarce. In the present study, we conducted a large-scale population-based analysis to investigate the long-term outcomes of renal recipients with HCV infection. Propensity score matching with a ratio of 1:1 was applied. A total of 6,473renal recipients with HCV infection in case group were enrolled after PSM. Our findings showed that subjects with HCV infection in kidney transplant had significantly higher risk of hepatoma, cirrhosis, hepatic failure, and overall hepatic disease than those without HCV infection. (hepatoma: HR: 8.957; 95% CI: 5.324-15.069; cirrhosis: HR: 5.378; 95% CI: 4.363-6.631; hepatic failure: HR: 3.258; 95% CI: 2.527-4.200; overall hepatic disease: HR: 4.128; 95% CI: 3.428-4.971). In the present study, our findings show that renal recipients with HCV infection is significantly associated with a remarkably high risk of hepatic complications post-kidney transplantation.
Keywords: Dialysis; End-stage renal disease; Hepatic disease; Hepatitis C virus; Kidney transplantation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical statement: The study has received approval from the institutional review board (IRB) committee of Taichung Veterans General Hospital (approval number: SE22220A, TCVGH). The informed consent of the TriNetX database was waived by the Institutional Review Board of Taichung Veterans General Hospital (TCVGH) due to the anonymous nature of the database and its strict adherence to the guidelines stipulated by the Health Insurance Portability and Accountability Act and the General Data Protection Regulation (approval number: SE22220A, TCVGH).
Figures

References
-
- Saab, S., Martin, P., Brezina, M., Gitnick, G. & Yee, H. F. Jr. Serum Alanine aminotransferase in hepatitis c screening of patients on Hemodialysis. Am. J. Kidney Dis.37 (2), 308–315 (2001). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

