Afatinib amplifies cAMP-induced fluid secretion in a mouse mini-gut model via TMEM16A-mediated fluid secretion and secretory cell differentiation
- PMID: 40784986
- PMCID: PMC12336333
- DOI: 10.1038/s41598-025-14516-9
Afatinib amplifies cAMP-induced fluid secretion in a mouse mini-gut model via TMEM16A-mediated fluid secretion and secretory cell differentiation
Abstract
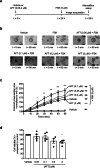
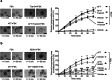
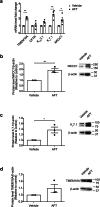
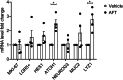
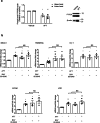
Afatinib is an effective treatment of metastatic non-small cell lung cancer, despite the occurrence of its common gastrointestinal toxicities especially diarrheas, which lead to dose adjustments or treatment cessation in severe cases. Underlying mechanisms of afatinib-induced diarrheas under prolonged treatment remain elusive. This study aimed to investigate mechanisms involved in the afatinib-induced fluid secretion in three-dimensional (3D) mouse mini-gut models under prolonged treatment (24 h). The swelling assay, qRT-PCR, and immunoblotting experiments were performed. Our results showed that afatinib amplified the cAMP-induced fluid secretion by 2 folds by mechanisms requiring TMEM16A and Kv7 channels via EPAC-calcium-dependent pathways. Additionally, afatinib treatment increased Kv7.1 and NKCC1 expression. Interestingly, afatinib induced secretory cell differentiation and upregulation of Paneth cell markers. Treatment with a PI3K inhibitor mimicked the effect of afatinib on increasing expression of membrane transporters and secretory lineage cell markers with no additive effect being observed after combination with afatinib, suggesting that the observed effect of afatinib was via PI3K inhibition. Collectively, our results indicate that prolonged afatinib treatment enhances cAMP-induced fluid secretion by mechanisms involving EPAC-TMEM16A-Kv7.1-mediated fluid secretion and secretory cell differentiation in 3D mouse mini-gut models. The EPAC-TMEM16A-Kv7.1-mediated fluid secretion represents a promising therapeutic target for treating afatinib-induced diarrheas.
Keywords: Afatinib; Diarrhea; Fluid secretion; Kv7.1; Paneth cell; TMEM16A.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Sartori, G. et al. Efficacy and safety of Afatinib for non-small-cell lung cancer: state-of-the-art and future perspectives. Expert Rev. Anticancer Ther.20 (7), 531–542 (2020). - PubMed
-
- Van Sebille, Y. Z., Gibson, R. J., Wardill, H. R. & Bowen, J. M. ErbB small molecule tyrosine kinase inhibitor (TKI) induced diarrhoea: chloride secretion as a mechanistic hypothesis. Cancer Treat. Rev.41 (7), 646–652 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

