FoxO3 Activation Alleviates Doxorubicin-Induced Cardiomyopathy by Enhancing Autophagic Flux and Suppressing mTOR/ROS Signalling
- PMID: 40785055
- PMCID: PMC12336054
- DOI: 10.1111/jcmm.70775
FoxO3 Activation Alleviates Doxorubicin-Induced Cardiomyopathy by Enhancing Autophagic Flux and Suppressing mTOR/ROS Signalling
Abstract
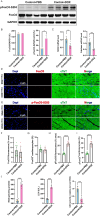
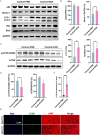
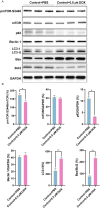
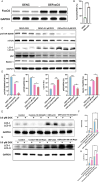
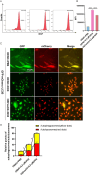
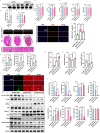
Doxorubicin (DOX) is an effective chemotherapy drug, but its use is limited by cardiotoxicity, known as DOX-induced cardiomyopathy. The transcription factor FoxO3, which regulates autophagy and oxidative stress, has unclear mechanisms in this condition. We found that DOX-induced cardiomyopathy involved cardiac atrophy, cardiac dysfunction, fibrosis and mitochondrial damage. DOX reduced H9c2 cardiomyocyte viability and glutathione levels (GSH), increased reactive oxygen species (ROS), malondialdehyde (MDA) and lactate dehydrogenase (LDH) and inhibited superoxide dismutase 2 (SOD2) and catalase (CAT) expression. DOX also suppressed FoxO3 activation and increased the autophagy protein LC3 II/I ratio. Overexpressing FoxO3 enhanced LC3B, Beclin 1 and autophagic flux, while reducing p62 and suppressing mTOR activation in heart. Brefeldin A1 (BafA1), an autophagy inhibitor and rapamycin (Rapa), an autophagy activator, were administered to H9c2 cardiomyocytes to elucidate the regulatory mechanism of FoxO3. Mechanically, our data revealed that FoxO3 overexpression enhanced autophagy and suppressed ROS production and mTOR activation in both in vitro and in vivo models of DOX exposure. Collectively, targeting FoxO3 to enhance protective autophagy may offer a therapeutic strategy against DOX-induced cardiomyopathy.
Keywords: FoxO3; autophagy; cardiomyopathy; doxorubicin; reactive oxygen species.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
MiR-24-3p modulates cardiac function in doxorubicin -induced heart failure via the Sp1/PI3K signaling pathway.Cell Signal. 2024 Dec;124:111407. doi: 10.1016/j.cellsig.2024.111407. Epub 2024 Sep 14. Cell Signal. 2024. PMID: 39278455
-
β-LAPachone ameliorates doxorubicin-induced cardiotoxicity via regulating autophagy and Nrf2 signalling pathways in mice.Basic Clin Pharmacol Toxicol. 2020 Apr;126(4):364-373. doi: 10.1111/bcpt.13340. Epub 2019 Nov 27. Basic Clin Pharmacol Toxicol. 2020. PMID: 31630478
-
Ring Finger Protein 2 Promotes Oxidative Stress and Mitochondrial Dysfunction in Doxorubicin-Induced Cardiotoxicity Via the Mercaptopyruvate Sulfurtransferase/Hydrogen Sulfide Pathway.J Am Heart Assoc. 2025 Aug 19;14(16):e041440. doi: 10.1161/JAHA.125.041440. Epub 2025 Aug 6. J Am Heart Assoc. 2025. PMID: 40767300
-
Cardioprotective strategies against doxorubicin-induced cardiotoxicity: A review from standard therapies to emerging mitochondrial transplantation.Biomed Pharmacother. 2025 Aug;189:118315. doi: 10.1016/j.biopha.2025.118315. Epub 2025 Jul 3. Biomed Pharmacother. 2025. PMID: 40614534 Review.
-
Autophagic dysregulation in doxorubicin cardiomyopathy.J Mol Cell Cardiol. 2017 Mar;104:1-8. doi: 10.1016/j.yjmcc.2017.01.007. Epub 2017 Jan 17. J Mol Cell Cardiol. 2017. PMID: 28108310 Review.
References
-
- Zamorano J. L., Lancellotti P., Rodriguez Munoz D., et al., “2016 ESC Position Paper on Cancer Treatments and Cardiovascular Toxicity Developed Under the Auspices of the ESC Committee for Practice Guidelines: The Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC),” European Journal of Heart Failure 19, no. 1 (2017): 9–42, 10.1002/ejhf.654. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 22A0528/Key Scientific Research Project of Hunan Provincial Department of Education
- 82202815/National Natural Science Foundation of China
- 2024KTSCX128/Characteristic Innovation Project of Guangdong Provincial Education Department
- SL2022A04J00488/Guangzhou Basic Research Plan, Basic and Applied Basic Research Project
- 2023JJ50252/Hunan Provincial Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

