The Role of Ethanol in Lithium-Mediated Nitrogen Reduction
- PMID: 40785206
- PMCID: PMC12371868
- DOI: 10.1021/jacs.5c03389
The Role of Ethanol in Lithium-Mediated Nitrogen Reduction
Abstract
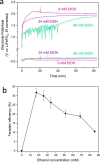
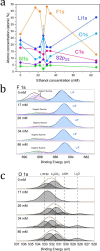
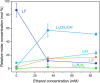
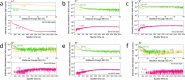
Although the Haber-Bosch process for industrial ammonia production is hailed by many as one of the most influential breakthroughs of the 20th century, its decarbonization and decentralization remain a critical challenge. One of the most promising and fast improving approaches is electrochemical nitrogen reduction mediated by lithium. However, the impact of electrolyte configuration on the formation of the solid electrolyte interphase (SEI) and its effect on selective nitrogen reduction is still elusive. In particular, the role of commonly added, supposedly sacrificial, proton donors on SEI chemistry and morphology remains a mystery. In this work, the impact of ethanol concentration in a 1 M LiNTf2 in THF electrolyte on SEI properties and nitrogen reduction is analyzed via a multipronged characterization approach. Post-mortem surface analysis via X-ray photoelectron spectroscopy shows a dependence in the relative proportion of LiF and Li2O on ethanol concentration, while depth profiling measurements via cluster source time-of-flight secondary ion mass spectrometry reveal increasing SEI electrolyte permeability at higher ethanol concentrations. Cryogenic electron microscopy measurements show a reduction in SEI thickness with increased ethanol concentration, as well as increased SEI homogeneity. Lithium metal is also observed only in the ethanol-free condition. Analysis of bulk SEI components via titration corroborates the observation of lithium metal in cryo-microscopy measurements, as well as showing an increase in bulk Li2-xOHx content with ethanol concentration. A narrow 'Goldilocks' region is revealed, where the SEI has just the right properties for efficient nitrogen reduction.
Figures


References
-
- Smith C., Hill A. K., Torrente-Murciano L.. Current and Future Role of Haber-Bosch Ammonia in a Carbon-Free Energy Landscape. Energy Environ. Sci. 2020;13(2):331–344. doi: 10.1039/C9EE02873K. - DOI
-
- MacFarlane D. R., Cherepanov P. V., Choi J., Suryanto B. H. R., Hodgetts R. Y., Bakker J. M., Ferrero Vallana F. M., Simonov A. N.. A Roadmap to the Ammonia Economy. Joule. 2020;4(6):1186–1205. doi: 10.1016/j.joule.2020.04.004. - DOI
-
- Chang W., Jain A., Rezaie F., Manthiram K.. Lithium-Mediated Nitrogen Reduction to Ammonia via the Catalytic Solid-Electrolyte Interphase. Nat. Catal. 2024;7(3):231–241. doi: 10.1038/s41929-024-01115-6. - DOI
-
- Smith C., Torrente-Murciano L.. The Potential of Green Ammonia for Agricultural and Economic Development in Sierra Leone. One Earth. 2021;4(1):104–113. doi: 10.1016/j.oneear.2020.12.015. - DOI
LinkOut - more resources
Full Text Sources

