Characterization of Breakthrough Hemolysis in Patients With Paroxysmal Nocturnal Hemoglobinuria: An International Multicenter Experience
- PMID: 40785544
- PMCID: PMC12516651
- DOI: 10.1002/ajh.70032
Characterization of Breakthrough Hemolysis in Patients With Paroxysmal Nocturnal Hemoglobinuria: An International Multicenter Experience
Abstract
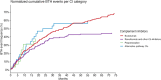
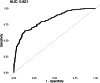
Breakthrough hemolysis (BTH) is defined as a hemolytic exacerbation in a patient with paroxysmal nocturnal hemoglobinuria (PNH) treated with complement inhibitors (CIs). In the current era of several terminal and proximal inhibitors, there are no guidelines for defining BTH and its severity, and clinical management is not standardized. This retrospective, observational, and multicentric study evaluated BTH frequency and severity in PNH patients treated with complement inhibitors from 2007 until February 2025 at 10 centers across Italy (9 centers) and the United Kingdom (1 center). A total of 271 BTH events occurred in 102 out of 198 patients (51%), with 36/198 (18%) patients experiencing 3 or more BTH episodes over the 18-year follow-up. Most events (55%) were prompted by infections, and 24 (10%) were linked to poor compliance with oral alternative pathway inhibitors. BTH was clinically severe in about half of patients, requiring transfusion in 46%, CI dosing adjustment in 17%, and anticoagulant prophylaxis in 16%. Overall, 5 (2%) breakthrough thrombotic events occurred in patients not on prophylaxis, all during an infection. BTH incidence was 0.19 events per patient-year, maximal with proximal inhibitors (0.4 events per patient-year), and lower for anti-C5 (0.18 per patient-year). By logistic regression analysis, the main predictors of BTH were more severe disease at diagnosis (increased LDH and a shorter time to first complement inhibitor), treatment with proximal inhibitors, and poorer EBMT response category.
Keywords: breakthrough hemolysis; danicopan; eculizumab; iptacopan; paroxysmal nocturnal hemoglobinuria; pegcetacoplan; ravulizumab; thrombosis.
© 2025 The Author(s). American Journal of Hematology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest related to the present publication. B.F., Sobi: Speakers Bureau; Samsung: Speakers Bureau; Novartis: Consultancy; Roche: Consultancy, Other: travel to congress; Alexion: Consultancy; Janssen: Consultancy; Agios: Research Funding; Zenas BioPharma: Research Funding. W.B., Alexion, AstraZeneca Rare Disease: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sobi: Consultancy; Sanofi: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau.
Figures
References
-
- Lee J. W., Brodsky R. A., Nishimura J. I., and Kulasekararaj A. G., “The Role of the Alternative Pathway in Paroxysmal Nocturnal Hemoglobinuria and Emerging Treatments,” Expert Review of Clinical Pharmacology 15, no. 7 (2022): 851–861. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

