Development and validation of a prognostic nomogram incorporating automated collateral score and computed tomography perfusion parameters for patients with acute ischemic stroke undergoing endovascular thrombectomy
- PMID: 40785896
- PMCID: PMC12332714
- DOI: 10.21037/qims-24-1956
Development and validation of a prognostic nomogram incorporating automated collateral score and computed tomography perfusion parameters for patients with acute ischemic stroke undergoing endovascular thrombectomy
Abstract
Background: Prognostic evaluation of patients with acute ischemic stroke (AIS) after endovascular thrombectomy (EVT) remains challenging. Traditional computed tomography angiography (CTA) and computed tomography perfusion (CTP) have limitations, whereas multiphase CTA (mCTA) allows for more accurate collateral circulation assessment. However, the predictive utility of automated collateral scoring remains underexplored. This study aimed to develop and validate a nomogram combining automated collateral scores and CTP parameters to enhance the prognostic prediction in patients with AIS undergoing EVT.
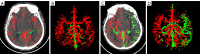
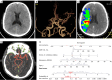
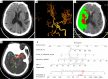
Methods: This retrospective study enrolled patients with AIS due to large-vessel occlusion (LVO) who underwent multimodal computed tomography (CT) and EVT between January 2017 and December 2022. The inclusion criteria were as follows: (I) age ≥18 years; (II) symptom onset within 24 hours; (III) available non-contrast CT (NCCT) and CTP; and (IV) 90-day modified Rankin Scale (mRS) score. Meanwhile, the exclusion criteria included posterior circulation stroke, intracranial hemorrhage, extensive prior infarction, prestroke mRS ≥2, poor image quality, and missing outcome data. A total of 111 patients were included and randomly assigned to development (n=77) and validation (n=34) cohorts. Clinical data, National Institutes of Health Stroke Scale (NIHSS) features, and imaging features [including automated collateral scores from NeuBrainCARE (NBC) software and relative cerebral blood flow (rCBF) <30% volume] were analyzed. Agreement between automated and manual Menon collateral scores was assessed via Cohen's kappa. A nomogram was constructed through multivariable logistic regression and validated with bootstrapping (500 iterations). Clinical outcomes were assessed at 90 days through the mRS, with outcomes categorized as good (mRS 0-2) or poor (mRS 3-6).
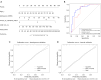
Results: Manual collateral scoring between physicians showed substantial agreement (κ=0.667). For the automated scoring system, the consistency with manual scoring performed by a junior physician resulted in a kappa value of 0.597, while comparison with senior physicians yielded a higher kappa value of 0.872. The median age was 67 years [interquartile range (IQR), 54-74] years, and 60.3% were male. At 90 days, 54.1% had good outcomes (mRS 0-2). Key independent predictors included age, admission NIHSS, automated collateral score, and rCBF <30% volume (P<0.05). The nomogram achieved strong discrimination in both the development [concordance index (C-index) 0.872, sensitivity 82.9%, and specificity 80.6%] and validation (C-index 0.807, sensitivity 68.4%, and specificity 93.3%) cohorts.
Conclusions: This study supports the use of automated collateral scoring as a reliable and unbiased method in AIS prognosis. The proposed nomogram, integrating clinical and imaging parameters, provides an effective tool for outcome prediction after EVT, facilitating individualized treatment planning and optimizing patient selection.
Keywords: Acute ischemic stroke (AIS); collateral status; computed tomography perfusion (CTP); endovascular thrombectomy (EVT); multiphase computed tomography angiography (mCTA).
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1956/coif). L.F. and Y.L. are currently employees of Neusoft Medical Systems Co., Ltd. All authors report the funding from Jiangsu Province Capability Improvement Project through Science, Technology and Education (Jiangsu Provincial Medical Key Discipline Cultivation Unit), China (No. JSDW202242); and Wuxi “Taihu Light” Science and Technology Research Project of Jiangsu Province, China (No. Y20232016). The authors have no other conflicts of interest to declare.
Figures


Similar articles
-
The influence of prestroke disability on outcome in patients with a low Alberta Stroke Program Early CT Score who underwent endovascular thrombectomy.J Neurosurg. 2025 Feb 7;143(1):266-273. doi: 10.3171/2024.10.JNS24888. Print 2025 Jul 1. J Neurosurg. 2025. PMID: 39919282
-
CT Perfusion Imaging After Selection for Late-Window Endovascular Stroke Treatment: Secondary Analysis of the MR CLEAN-LATE Randomized Trial.JAMA Neurol. 2025 Jun 1;82(6):589-596. doi: 10.1001/jamaneurol.2025.0716. JAMA Neurol. 2025. PMID: 40323620 Clinical Trial.
-
Deep Learning based Collateral Scoring on Multi-Phase CTA in patients with acute ischemic stroke in MCA region.AJNR Am J Neuroradiol. 2025 Jul 7:ajnr.A8911. doi: 10.3174/ajnr.A8911. Online ahead of print. AJNR Am J Neuroradiol. 2025. PMID: 40623825
-
Endovascular thrombectomy with versus without intravenous thrombolysis for acute ischaemic stroke.Cochrane Database Syst Rev. 2025 Apr 24;4(4):CD015721. doi: 10.1002/14651858.CD015721.pub2. Cochrane Database Syst Rev. 2025. PMID: 40271574
-
Good collaterals and better outcomes after EVT for basilar artery occlusion: A systematic review and meta-analysis.Int J Stroke. 2023 Oct;18(8):917-926. doi: 10.1177/17474930231154797. Epub 2023 Feb 2. Int J Stroke. 2023. PMID: 36655949
References
-
- Rodrigues M, Cunha A, Figueiredo S, Carvalho A, Veloso M, Barros P, Gregório T, Paredes L, Pinho J, Castro S, Ribeiro M. Emergent carotid artery stenting in atherosclerotic disease of the internal carotid artery with tandem intracranial occlusion. J Neurol Sci 2018;387:196-8. 10.1016/j.jns.2018.02.034 - DOI - PubMed
-
- García-Tornel Á, Campos D, Rubiera M, Boned S, Olivé-Gadea M, Requena M, Ciolli L, Muchada M, Pagola J, Rodriguez-Luna D, Deck M, Juega J, Rodríguez-Villatoro N, Sanjuan E, Tomasello A, Piñana C, Hernández D, Álvarez-Sabin J, Molina CA, Ribó M. Ischemic Core Overestimation on Computed Tomography Perfusion. Stroke 2021;52:1751-60. 10.1161/STROKEAHA.120.031800 - DOI - PubMed
LinkOut - more resources
Full Text Sources
