Impact of anatomical variations of the circle of Willis on the blood flow within unruptured intracranial aneurysm
- PMID: 40785920
- PMCID: PMC12332671
- DOI: 10.21037/qims-2025-55
Impact of anatomical variations of the circle of Willis on the blood flow within unruptured intracranial aneurysm
Abstract
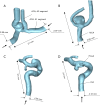
Background: Anatomical variations of the circle of Willis (CoW) are closely associated with the occurrence of intracranial aneurysms (IAs). However, the impact of anatomical variations on the rupture risk of IAs remains unclear. The purpose of this study was to explore the effect of artery absence on the internal flow and rupture risk of IAs.
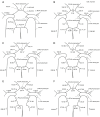
Methods: A one-dimensional (1D) solver was combined with a 3-dimensional (3D) fluid-structure interaction (FSI) model to effectively quantify the hemodynamic characteristics inside IA under artery absence.
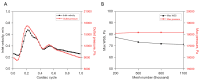
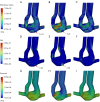
Results: The 1D results showed that the absence of anterior cerebral artery A1 segment (ACA-A1) or posterior cerebral artery P1 segment (PCA-P1) will trigger a compensatory blood flow effect, leading to significant blood flow variations of the anterior communicating artery (ACoA) and internal carotid artery (ICA). By FSI calculation, in the absence of ACA-A1, the maximum wall shear stress (WSS) within the ACoA aneurysm increased by 103% or more compared to the complete CoW due to blood inflow jet. In addition, WSS increased by 45% and 12% in the contralateral ICA and posterior communicating artery (PCoA) aneurysm respectively, whereas it decreased by 33% and 35% in the ipsilateral ICA and PCoA aneurysm, respectively. The absence of PCA-P1 had a less significant impact on the global blood flow of the CoW compared to the absence of ACA-A1, but it still led to an increase in WSS within the ipsilateral ICA and PCoA aneurysms (25% and 22%, respectively).
Conclusions: The absence of ACA-A1 or PCA-P1 may serve as an IA rupture risk factor. If ACA-A1 or PCA-P1 absence is identified clinically alongside an aneurysm, proactive treatment strategies are advised.
Keywords: Intracranial aneurysm (IA); anatomical variations; circle of Willis (CoW); fluid-structure interaction (FSI).
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2025-55/coif). The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Variations in intracranial arterial anatomy of the circle of Willis and their association with arteriosclerosis in patients with ischemic cerebrovascular disease.Int J Stroke. 2025 Aug;20(7):843-851. doi: 10.1177/17474930251322678. Epub 2025 Feb 27. Int J Stroke. 2025. PMID: 39924650 Free PMC article.
-
Hemodynamics of unruptured and ruptured middle cerebral artery aneurysms based on fluid-structure interaction.J Clin Neurosci. 2025 Jul 10;139:111433. doi: 10.1016/j.jocn.2025.111433. Online ahead of print. J Clin Neurosci. 2025. PMID: 40645151
-
Patterns of Dynamic Adaptability of the Circle of Willis in Response to Major Branch Artery Coverage With a Flow Diverter.World Neurosurg. 2025 Jan;193:1065-1075. doi: 10.1016/j.wneu.2024.10.044. Epub 2024 Nov 9. World Neurosurg. 2025. PMID: 39433250
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
-
Automated devices for identifying peripheral arterial disease in people with leg ulceration: an evidence synthesis and cost-effectiveness analysis.Health Technol Assess. 2024 Aug;28(37):1-158. doi: 10.3310/TWCG3912. Health Technol Assess. 2024. PMID: 39186036 Free PMC article.
References
-
- Greving JP, Wermer MJ, Brown RD, Jr, Morita A, Juvela S, Yonekura M, Ishibashi T, Torner JC, Nakayama T, Rinkel GJ, Algra A. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 2014;13:59-66. 10.1016/S1474-4422(13)70263-1 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
