Deciphering Mitochondria: Unveiling Their Roles in Mechanosensing and Mechanotransduction
- PMID: 40785969
- PMCID: PMC12332263
- DOI: 10.34133/research.0816
Deciphering Mitochondria: Unveiling Their Roles in Mechanosensing and Mechanotransduction
Abstract
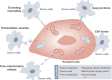
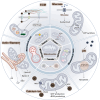
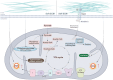
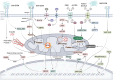
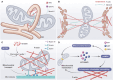
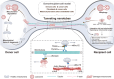
Mitochondria are highly dynamic organelles that are responsible for essential cellular functions such as calcium regulation, reactive oxygen species (ROS) production, metabolism, and apoptosis initiation. Mitochondrial dysfunctions are associated with a variety of pathologies, and the onset and progression of disease are accompanied by alterations in extracellular biochemical and mechanical signals. Recent studies have demonstrated that physicochemical cues, especially mechanical cues, exert pivotal roles in the organization of mitochondrial network and their metabolic functions. Therefore, understanding the mechanisms that orchestrate mitochondrial morphology and function is essential for elucidating their role in both health and disease. This review discusses novel insights into the recent advances regarding mitochondrial dysfunction across a spectrum of diseases and describes the effect of various factors. It then highlights the recently discovered mechanisms, particularly those involving matrix mechanical cues and cellular mechanical cues, summarizing the multiple pathways of mechanotransduction, such as integrin, Piezo1/TRPV4, and YAP/TAZ signaling pathways. Last, the review explores the potential future directions, stressing that understanding mitochondrial dysfunction is crucial for developing effective therapies to improve mitochondrial function and address related diseases.
Copyright © 2025 Jiaxuan Yu et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures

References
-
- Shen K, Pender CL, Bar-Ziv R, Zhang H, Wickham K, Willey E, Durieux J, Ahmad Q, Dillin A. Mitochondria as cellular and organismal signaling hubs. Annu Rev Cell Dev Biol. 2022;38:179–218. - PubMed
-
- Chan DC. Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol. 2020;15:235–259. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

