RETRACTED ARTICLE: Generation of long-chain fatty acids by hydrogen-driven bicarbonate reduction in ancient alkaline hydrothermal vents
- PMID: 40785984
- PMCID: PMC12331513
- DOI: 10.1038/s43247-023-01196-4
RETRACTED ARTICLE: Generation of long-chain fatty acids by hydrogen-driven bicarbonate reduction in ancient alkaline hydrothermal vents
Abstract
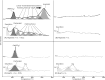
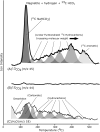
The origin of life required membrane-bound compartments to allow the separation and concentration of internal biochemistry from the external environment and establish energy-harnessing ion gradients. Long-chain amphiphilic molecules, such as fatty acids, appear strong candidates to have formed the first cell membranes although how they were first generated remains unclear. Here we show that the reaction of dissolved hydrogen and bicarbonate with the iron-rich mineral magnetite under conditions of continuous flow, alkaline pH and relatively low temperatures (90 °C) generate a range of functionalised long-chain aliphatic compounds, including mixed fatty acids up to 18 carbon atoms in length. Readily generated membrane-forming amphiphilic organic molecules in the first cellular life may have been driven by similar chemistry generated from the mixing of bicarbonate-rich water (equilibrated with a carbon dioxide-enriched atmosphere) with alkaline hydrogen-rich fluids fed by the serpentinisation of the Earth's iron-rich early crust.
Keywords: Biogeochemistry; Geochemistry; Mineralogy; Origin of life.
© The Author(s) 2024.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures


Similar articles
-
Comparison of cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease.Cochrane Database Syst Rev. 2001;(3):CD003234. doi: 10.1002/14651858.CD003234. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003234. doi: 10.1002/14651858.CD003234.pub2. PMID: 11687058 Updated.
-
Conversion of pyridoxal to pyridoxamine with NH3 and H2 on nickel generates a protometabolic nitrogen shuttle under serpentinizing conditions.FEBS J. 2025 Jun;292(12):3041-3055. doi: 10.1111/febs.17357. Epub 2024 Dec 19. FEBS J. 2025. PMID: 39703002 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Bio-mediated CN cycling in serpentinites and the origin of life.Sci Rep. 2025 Jul 1;15(1):22452. doi: 10.1038/s41598-025-04161-7. Sci Rep. 2025. PMID: 40593967 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
References
-
- Gill, S. & Forterre, P. Origin of life: LUCA and extracellular membrane vesicles (EMVs). Int. J. Astrobiol.15, 7–15 (2016).
-
- Deamer, D. Membranes and the origin of life: a century of conjecture. J. Mol. Evol.83, 159–168 (2016). - PubMed
-
- Lane, N. Proton gradients at the origin of life. BioEssays39, 1600217–1600217 (2017). - PubMed
-
- Lane, N. Why are cells powered by proton gradients? Nat. Educ.3, 2 (2010).
LinkOut - more resources
Full Text Sources
