Impact of Methicillin-Resistant Staphylococcus aureus Nasal PCR Versus Culture on Vancomycin Utilization in Pneumonia Management
- PMID: 40786007
- PMCID: PMC12328358
- DOI: 10.1177/87551225251359508
Impact of Methicillin-Resistant Staphylococcus aureus Nasal PCR Versus Culture on Vancomycin Utilization in Pneumonia Management
Abstract
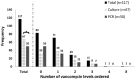
Introduction: Methicillin-resistant Staphylococcus aureus pneumonia (PNA) can be ruled out via methicillin-resistant Staphylococcus aureus (MRSA) culture and polymerase chain reaction (PCR) nasal screening, facilitating the de-escalation of empiric anti-MRSA agents like intravenous vancomycin. This study evaluated the impact of transitioning from culture to PCR-based MRSA nasal screening in patients with PNA. Methods: This Institutional Review Board (IRB)-approved retrospective quasi-experimental study was conducted at a 5-hospital system and included adult, nonpregnant hospitalized patients from September to December 2021 ("culture group") and September to December 2022 ("PCR group") and diagnosed with PNA. Exclusion criteria were ventilator-acquired PNA or positive MRSA respiratory culture. The primary endpoint was the number of vancomycin levels obtained. Secondary endpoints were vancomycin duration as well as acute kidney injury (AKI) and all-cause 30-day readmission rates. Results: Two-hundred patients were included: 100 in each group. Baseline characteristics were similar. There were 117 vancomycin levels obtained: 67 (67) and 50 (50) in the culture and PCR group, respectively (P = .021). Median vancomycin duration was 50% shorter in the PCR group: 2 days (1-3) versus 3 days (2-4), P < .001. After adjusting for confounders, the culture group was more likely to have vancomycin levels obtained compared to the PCR group: adjusted odd ratio (aOR) (95% confidence interval [CI])] = 1.833 (1.016-3.309). Long-term obstructive pulmonary disease was associated with reduced risk of ordering vancomycin levels: aOR [95% CI] = 0.426 (0.218-0.831). Readmission and AKI rates were comparable. Conclusion: Transitioning from culture to PCR-based MRSA nasal screening significantly reduced vancomycin levels obtained from patients and shortened vancomycin duration without negatively impacting patient outcome.
Keywords: MRSA; PCR; culture; swab; vancomycin.
© The Author(s) 2025.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Meng L, Pourali S, Hitchcock MM, et al. Discontinuation patterns and cost avoidance of a pharmacist-driven methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction testing protocol for de-escalation of empiric vancomycin for suspected pneumonia. Open Forum Infect Dis. 2021;8(4):ofab099. doi: 10.1093/ofid/ofab099 - DOI - PMC - PubMed
-
- Evans W, Paciullo K, Trible R. Pharmacist-driven methicillin-resistant Staphylococcus aureus screening protocol and the impact on vancomycin exposure in hospitalized patients with pneumonia. J Am Coll Clin Pharm. 2021;4(12):1548-1553. doi: 10.1002/jac5.1530 - DOI
LinkOut - more resources
Full Text Sources