A glycogen derived from sea urchin- Strongylocentyotus internedius shifts macrophages to the M1 phenotype and enhances the anti-pancreatic cancer activity of gemcitabine
- PMID: 40786029
- PMCID: PMC12331687
- DOI: 10.3389/fphar.2025.1600349
A glycogen derived from sea urchin- Strongylocentyotus internedius shifts macrophages to the M1 phenotype and enhances the anti-pancreatic cancer activity of gemcitabine
Abstract
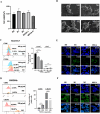
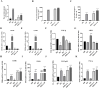
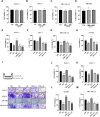
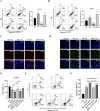
One of the biggest obstacles to treating pancreatic ductal adenocarcinoma (PDAC) is chemotherapy resistance. Macrophages are an essential element of the innate immune system and are distributed in almost every tissue in the body. Among them, macrophages infiltrating into the tumor microenvironment negatively regulate tumor immunity and participate in the generation, invasion, migration and drug resistance of PDAC. In prior study, we isolated a polysaccharide from sea urchin-Strongylocentyotus internedius, which was identified as a high molecular weight, highly branched glycogen (MSGA). In this study, we found that MSGA increased the expression of iNOS, IL-6, TNFα, IL-12 and triggered macrophage differentiation to the CD86+ M1 phenotype. MSGA-induced M1 macrophages decreased the cell viabilities and induced apoptosis of PDAC cells. When combined with gemcitabine (GEM), MSGA significantly enhanced the pro-apoptotic activity of GEM. Mechanistically, MSGA transformed macrophages to the M1 phenotype through the stimulation of the JAK1/3-STAT1 signaling pathway and the suppression of STAT3 activity. Overall, our research showed that MSGA has profound potential for tumor immunotherapy. And as an "immune stimulator", MSGA could assist GEM in the treatment of PDAC.
Keywords: JAK; STAT signaling pathway; gemcitabine; glycogen; macrophages; tumor microenvironment.
Copyright © 2025 Deng, Yu, Wu, Wang, Wang, Yue, Geng and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Catenacci D. V. T., Junttila M. R., Karrison T., Bahary N., Horiba M. N., Nattam S. R., et al. (2015). Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. Clin. Oncol. 33, (36), 4284-4292. 10.1200/jco.2015.62.8719 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

