Assessment of drug-related migraine in a real-world large-scale database
- PMID: 40786030
- PMCID: PMC12332265
- DOI: 10.3389/fphar.2025.1647088
Assessment of drug-related migraine in a real-world large-scale database
Abstract
Background: Drug-induced migraine represents a clinically significant yet under-investigated subtype of migraine. This study aims to evaluate the risk of drug-related migraine based on real-world data from the FDA Adverse Event Reporting System (FAERS).
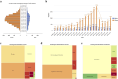
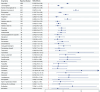
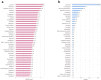
Methods: A retrospective pharmacovigilance analysis was conducted using FAERS data from Q1 2004 to Q4 2024. Migraine cases were identified via standardized MedDRA (The Medical Dictionary for Regulatory Activities) terms. Only primary suspect drugs were included. Disproportionality analyses were performed using four algorithms: ROR, PRR, MGPS, and BCPNN. Drugs were classified by therapeutic indication and mechanism of action, and stratified by BCPNN values to assess risk levels.
Results: A total of 20,886 migraine-related adverse events were identified, predominantly among females (77.4%) with a mean age of 45.7 years. Sixty-six drugs yielded positive signals, and after exclusion criteria, 39 remained for further analysis. The highest-risk agents included lorcaserin (BCPNN = 3.33), tasimelteon (3.20), and botulinum toxin type A (3.06). High-risk therapeutic classes included immunosuppressants, estrogens/progestogens, and sedative-hypnotics.
Conclusion: This large-scale analysis identifies key drug categories and compounds associated with an elevated risk of migraine, providing actionable insights for clinicians. Especially lorcaserin, tasimelteon, and botulinum toxin as potential risk factors for migraine. Given the public health burden of migraine, pharmacovigilance efforts should incorporate such findings to mitigate iatrogenic risks. Further prospective studies are warranted to establish causal mechanisms and optimize therapeutic decision-making.
Keywords: BCPNN; FAERS; disproportionality analysis; drug-induced headache; migraine; pharmacovigilance.
Copyright © 2025 Wu, Liu, Jiang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Sex differences in drug-induced osteoporosis: a pharmacovigilance study based on the FAERS database.Front Public Health. 2025 Jul 24;13:1630412. doi: 10.3389/fpubh.2025.1630412. eCollection 2025. Front Public Health. 2025. PMID: 40777658 Free PMC article.
-
A pharmacovigilance study of vortioxetine based on data from the FDA adverse event reporting system.Sci Rep. 2025 Aug 7;15(1):28886. doi: 10.1038/s41598-025-13786-7. Sci Rep. 2025. PMID: 40775011 Free PMC article.
-
Safety evaluation of irinotecan: a real-world disproportionality analysis using FAERS and JADER databases during the time period 2004-2024.Front Pharmacol. 2025 Jun 9;16:1516449. doi: 10.3389/fphar.2025.1516449. eCollection 2025. Front Pharmacol. 2025. PMID: 40552159 Free PMC article.
-
Adverse drug reactions related to methotrexate: a real-world pharmacovigilance study using the FAERS database from 2004 to 2024.Front Immunol. 2025 Jun 4;16:1586361. doi: 10.3389/fimmu.2025.1586361. eCollection 2025. Front Immunol. 2025. PMID: 40534848 Free PMC article. Review.
-
Botulinum toxins for the prevention of migraine in adults.Cochrane Database Syst Rev. 2018 Jun 25;6(6):CD011616. doi: 10.1002/14651858.CD011616.pub2. Cochrane Database Syst Rev. 2018. PMID: 29939406 Free PMC article.
References
-
- Arreola-Espino R., Urquiza-Marín H., Ambriz-Tututi M., Araiza-Saldaña C. I., Caram-Salas N. L., Rocha-González H. I., et al. (2007). Melatonin reduces formalin-induced nociception and tactile allodynia in diabetic rats. Eur. J. Pharmacol. 577 (1–3), 203–210. 10.1016/j.ejphar.2007.09.006 - DOI - PubMed
LinkOut - more resources
Full Text Sources

