Piperine protects against cerebral ischemic injury by regulating the Caspase-1-mediated pyroptosis pathway
- PMID: 40786035
- PMCID: PMC12332512
- DOI: 10.3389/fphar.2025.1601873
Piperine protects against cerebral ischemic injury by regulating the Caspase-1-mediated pyroptosis pathway
Abstract
Background: Ischemic stroke (IS) is a prevalent form of stroke and marked by high rates of morbidity, disability, and mortality. IS greatly threatens the physical health of people around the world. Oxidative stress triggered by IS can lead to inflammatory responses. Piperine (Pip) is a bioactive dietary phytochemical known for its pharmacological properties, including anti-inflammatory, anti-tumor, and antioxidant effects. Pip has attracted considerable interest among researchers. This study aims to investigate whether Pip attenuates cerebral ischemic injury by regulating the Caspase-1-mediated pyroptosis pathway.
Methods: In vivo and in vitro experimental models were employed. For the in vivo simulation of cerebral ischemia, the rat permanent middle cerebral artery occlusion (pMCAO) model was utilized. For the in vitro simulation, the BV-2 cells were subjected to oxygen-glucose deprivation (OGD). The recovery of neurological function in rats was assessed through multiple behavioral tests, including the Zea-Longa score, balance beam test, traverse beam test, forelimb grip pull test, postural reflex test, sensory test, and tail lifting test. Pathological changes in cerebral ischemic injury were observed using TTC staining, HE staining, and transmission electron microscopy. In in vivo and in vitro experiments, the potential protective mechanism of Pip in alleviating cerebral ischemic injury by regulating the Caspase-1-mediated pyroptosis pathway was investigated using Western blot and reverse transcription-polymerase chain reaction assays.
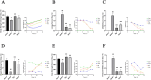
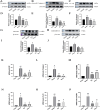
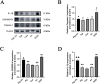
Results: In the in vivo experiments, compared with the Sham group, the Model group exhibited significant neurological damage, increased infarct volume, brain tissue edema, and elevated protein and mRNA expression levels of pyroptosis-associated factors. By contrast, the Pip group demonstrated notable improvements in behavioral function, brain tissue morphology, and the expression levels of pyroptosis-related factors compared with the Model group. In the in vitro experiments, the protein and mRNA expression of pyroptosis-associated factors in the OGD group were significantly upregulated compared with that in the Con group. However, the expression of these factors in the OGD+Pip group was markedly reduced compared with that in the OGD group. Furthermore, when cells were treated with the Caspase-1 inhibitor Ac-YVAD-cmk, the results revealed a significant decrease in the protein expression of Caspase-1 and its downstream factors, GSDMD-N and IL-1β, compared with that in the OGD group. Notably, the protein expression of GSDMD-N and IL-1β in the Pip+Ac-YVAD-cmk group was significantly higher than in the Pip group, which suggests that the inhibition of Caspase-1 attenuated the suppressive effect of Pip on GSDMD-N and IL-1β expression.
Conclusion: Pip exerts neuroprotective effects by modulating the Caspase-1-mediated pyroptosis pathway, which inhibits neuronal damage in the pMCAO model. These findings highlight the therapeutic potential of Pip in mitigating cerebral ischemic injury.
Keywords: Caspase-1; cerebral ischemic injury; natural product; neuroprotective; pyroptosis.
Copyright © 2025 Lu, Dai, Xi, Wang, Zhang, Fu, Liu and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Remote Ischemic Postconditioning Improve Cerebral Ischemia-Reperfusion Injury Induced Cognitive Dysfunction through Suppressing Mitochondrial Apoptosis in Hippocampus via TK/BK/B2R-Mediated PI3K/AKT.Mol Neurobiol. 2025 Aug;62(8):10652-10669. doi: 10.1007/s12035-025-04864-y. Epub 2025 Apr 14. Mol Neurobiol. 2025. PMID: 40229456 Free PMC article.
-
Ursolic acid ameliorates cerebral ischemia-reperfusion injury by inhibiting NF-κB/NLRP3-mediated microglia pyroptosis and neuroinflammation.Front Pharmacol. 2025 Jul 11;16:1622131. doi: 10.3389/fphar.2025.1622131. eCollection 2025. Front Pharmacol. 2025. PMID: 40717974 Free PMC article.
-
14,15-EET Maintains Mitochondrial Homeostasis to Inhibit Neuronal Pyroptosis After Ischemic Stroke.Stroke. 2025 Jul;56(7):1883-1896. doi: 10.1161/STROKEAHA.124.049143. Epub 2025 Apr 16. Stroke. 2025. PMID: 40235438
-
Downregulation of Nogo-B ameliorates cerebral ischemia/reperfusion injury in mice through regulating microglia polarization via TLR4/NF-kappaB pathway.Neurochem Int. 2023 Jul;167:105553. doi: 10.1016/j.neuint.2023.105553. Epub 2023 May 23. Neurochem Int. 2023. PMID: 37230196 Review.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

