Chaihu Longgu Muli Decoction inhibits chronic stress-induced lung cancer epithelial-mesenchymal transition process by suppressing Rap1/ERK signal pathway
- PMID: 40786046
- PMCID: PMC12332513
- DOI: 10.3389/fphar.2025.1644315
Chaihu Longgu Muli Decoction inhibits chronic stress-induced lung cancer epithelial-mesenchymal transition process by suppressing Rap1/ERK signal pathway
Abstract
Background: Chaihu Longgu Muli Decoction (CLM) is a classical herbal formula originally documented in Shang Han Lun. With an 1800-year clinical history, CLM remains widely prescribed for depression ("Yu Zheng" in Traditional Chinese Medicine theory). Emerging evidence suggests that chronic stress-induced depression is closely linked to lung cancer progression and metastasis. However, the therapeutic potential of CLM in this context remains unexplored.
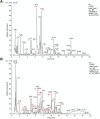
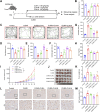
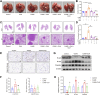
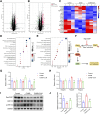
Methods: A lung cancer cell xenograft model combined with chronic unpredictable mild stress (CUMS) was used to evaluate the effect of CLM on lung cancer growth. Proteomic analysis was performed to explore the underlying mechanisms by which CLM alleviates CUMS-induced lung cancer progression. Western blot and qPCR were conducted to detect changes in Rap1/ERK-mediated epithelial-mesenchymal transition (EMT) progression. Finally, Rap1 agonists were utilized to determine the therapeutic mechanism of CLM on cortisol or corticosterone (Cort)-induced EMT progression in lung cancer cells and a mouse lung cancer model.
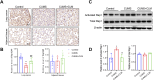
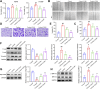
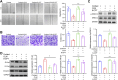
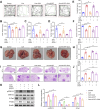
Results: In our study, CUMS promoted lung cancer xenograft growth, increased the expression of the proliferation marker Ki67, and elevated serum Cort levels. CLM treatment not only alleviated CUMS-induced depression-like behaviors, but also suppressed stress-driven tumor growth. These effects were replicated in a urethane-induced lung cancer model combined with CUMS. Proteomic analysis revealed that CLM's anti-tumor effects were associated with modulation of the Rap1 pathway. Mechanistically, CUMS downregulated Rap1GAP, activating Rap1 and subsequent ERK1/2 phosphorylation, thereby promoting EMT in lung cancer tissues. CLM effectively reversed these effects by inhibiting Rap1/ERK-mediated EMT. In vitro, CLM suppressed cortisol-induced migration, invasion, and EMT in lung cancer cells, and these effects were attenuated by Rap1 agonists. Furthermore, CLM inhibited Cort-induced EMT and depression-like behaviors in vivo, while Rap1 activation diminished CLM's efficacy against Cort-driven tumor growth.
Conclusion: These findings suggest that Rap1/ERK-mediated EMT is a hallmark of chronic stress-associated lung cancer progression. CLM exerts its therapeutic effects by targeting this pathway, offering a novel strategy to mitigate stress-aggravated oncogenesis.
Keywords: Chaihu Longgu Muli Decoction; Rap1; chronic stress; epithelial-mesenchymal transition; lung cancer.
Copyright © 2025 Li, Huang, Wang, Guo, Wang, Li and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Da-yuan-yin decoction alleviates bleomycin-induced pulmonary injury by inhibiting epithelial-mesenchymal transition via E-cadherin/β-catenin complex restoration.J Ethnopharmacol. 2025 Jul 24;351:120148. doi: 10.1016/j.jep.2025.120148. Epub 2025 Jun 14. J Ethnopharmacol. 2025. PMID: 40523450
-
Efficacy and safety of Chaihu plus Longgu Muli decoction combined with Estazolam in the treatment of insomnia: a meta-analysis.Sleep Breath. 2025 Jun 28;29(4):229. doi: 10.1007/s11325-025-03404-1. Sleep Breath. 2025. PMID: 40581702 Free PMC article. Review.
-
YiQiChuTan formula (YQCTF) inhibit the progression of non-small cell lung cancer via down-regulating EGFR/ITGB2 signaling: Triangulated evidence from network pharmacology, proteomic profiling, and experimental validation.Phytomedicine. 2025 Aug;144:156950. doi: 10.1016/j.phymed.2025.156950. Epub 2025 Jun 4. Phytomedicine. 2025. PMID: 40505482
-
Caveolin-1 inhibits the proliferation and invasion of lung adenocarcinoma via EGFR degradation.Sci Rep. 2025 Jul 1;15(1):21654. doi: 10.1038/s41598-025-05259-8. Sci Rep. 2025. PMID: 40594106 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- An Y., Liu Z., Wang S., Wang Q., Zhang C., Zhang L., et al. (2022). Effect of chaihu plus longgu muli decoction plus five-element music therapy in the treatment of cancer-related depression. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 30, 7955–7962. 10.1007/s00520-022-07172-6 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

