Comparative long-term outcomes of first-line CDK4/6 inhibitors plus endocrine therapy versus endocrine therapy in patients with HR+/HER2-metastatic or advanced breast cancer: a meta-analysis
- PMID: 40786051
- PMCID: PMC12331727
- DOI: 10.3389/fphar.2025.1600892
Comparative long-term outcomes of first-line CDK4/6 inhibitors plus endocrine therapy versus endocrine therapy in patients with HR+/HER2-metastatic or advanced breast cancer: a meta-analysis
Abstract
Introduction: This meta-analysis was designed to compare the long-term outcomes of first-line cyclin-dependent kinase 4/6 (CDK4/6) inhibitors plus endocrine therapy (ET) versus ET in patients with HR+/HER2-metastatic or advanced breast cancer (BC).
Materials and methods: Four databases (Medline, Embase, Web of Science, and CENTRAL) were searched for literature comparing First-line CDK4/6 inhibitors plus ET to ET in patients with HR+/HER2-metastatic or advanced breast cancer. The search was conducted from the database's establishment to April 3, 2025. Progression-free survival (PFS), overall survival (OS), objective response rate (ORR), disease control rate (DCR), and severe treatment-related adverse events (SAEs) were subjected to meta-analyses.
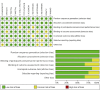
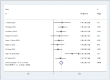
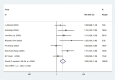
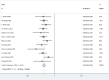
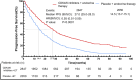
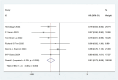
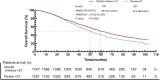
Results: Twelve Randomized controlled trials (RCTs) were included in the meta-analysis. The meta-analysis included a group of 4,957 patients diagnosed with HR+/HER2-metastatic or advanced breast cancer. Within this cohort, 2,877 patients were administered First-line CDK4/6 inhibitors together with ET, while 2080 patients received first-line ET alone. Compared with ET, First-line CDK4/6 inhibitors plus ET yielded superior ORR (RR = 1.39, 95% CI, 1.28 to 1.51, P < 0.01), DCR (RR = 1.09, 95% CI, 1.05 to 1.14, P < 0.01), PFS (HR: 0.57, 95%CI 0.53 to 0.62, P < 0.01) and OS (HR: 0.81, 95%CI 0.73 to 0.89, P < 0.01). Reconstruction of Kaplan-Meier curves for OS and PFS using the IPDformKM program provided a clear and comprehensible representation of oncological outcomes. First-line CDK4/6 inhibitors plus ET was more effective than ET in terms of PFS (median survival time: 27.0 months versus 14.4 months, HR: 0.55, 95%CI 0.51 to 0.59, P < 0.01) and OS (median survival time: 59.6 months versus 50.0 months, HR: 0.79, 95%CI 0.72 to 0.87, P < 0.01). With regards to safety, First-line CDK4/6 inhibitors plus ET exhibited a greater likelihood of encountering SAEs (RR = 1.54, 95% CI: 1.30 to 1.82, P < 0.01) in comparison to ET.
Conclusion: The present meta-analysis reported comparative long-term outcomes of CDK4/6 inhibitors plus ET versus ET as first-line therapy for HR+/HER2-metastatic or advanced breast cancer. Compared with ET alone, CDK4/6 inhibitors plus ET as first-line therapy provided improved ORR, DCR, PFS, and OS. Furthermore, the heightened efficacy of CDK4/6 inhibitors plus ET was accompanied by a rise in SAEs.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024590572, Identifier CRD42024590572.
Keywords: CDK4/6 inhibitors; breast cancer; endocrine therapy; meta-analysis; metastatic; objective response rate; overall survival; progression-free survival.
Copyright © 2025 Wang, Liang, Li, Qin, Wu, Qin, Zou, Zeng, Li, Huang, Tao, Quan and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
First-line therapy for adults with advanced renal cell carcinoma: a systematic review and network meta-analysis.Cochrane Database Syst Rev. 2023 May 4;5(5):CD013798. doi: 10.1002/14651858.CD013798.pub2. Cochrane Database Syst Rev. 2023. PMID: 37146227 Free PMC article.
-
Survival Following CDK4/6 Inhibitor Therapy for Hormone Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer.JAMA Netw Open. 2025 Feb 3;8(2):e2461067. doi: 10.1001/jamanetworkopen.2024.61067. JAMA Netw Open. 2025. PMID: 39982725 Free PMC article.
-
Cost-effectiveness of CDK4/6 inhibitors in HR+/HER2- metastatic breast cancer: a systematic review and meta-analysis.Curr Med Res Opin. 2024 Oct;40(10):1753-1767. doi: 10.1080/03007995.2024.2402074. Epub 2024 Sep 21. Curr Med Res Opin. 2024. PMID: 39305463
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Influence of ethnicity on cyclin-dependent kinase inhibitor efficacy and toxicity: A systematic review and meta-analysis.Breast. 2025 Feb;79:103833. doi: 10.1016/j.breast.2024.103833. Epub 2024 Nov 4. Breast. 2025. PMID: 39579620 Free PMC article.
References
-
- Adkins D., Ley J., Neupane P., Worden F., Sacco A. G., Palka K., et al. (2019). Palbociclib and cetuximab in platinum-resistant and in cetuximab-resistant human papillomavirus-unrelated head and neck cancer: a multicentre, multigroup, phase 2 trial. Lancet Oncol. 20 (9), 1295–1305. 10.1016/s1470-2045(19)30405-x - DOI - PubMed
-
- Albanell J., Martínez M. T., Ramos M., O'Connor M., de la Cruz-Merino L., Santaballa A., et al. (2022). Randomized phase II study of fulvestrant plus palbociclib or placebo in endocrine-sensitive, hormone receptor-positive/HER2–advanced breast cancer: GEICAM/2014–12 (FLIPPER). Eur. J. Cancer 161, 26–37. 10.1016/j.ejca.2021.11.010 - DOI - PubMed
-
- Altucci L., Addeo R., Cicatiello L., Germano D., Pacilio C., Battista T., et al. (1997). Estrogen induces early and timed activation of cyclin-dependent kinases 4, 5, and 6 and increases cyclin messenger ribonucleic acid expression in rat uterus. Endocrinology 138 (3), 978–984. 10.1210/endo.138.3.5002 - DOI - PubMed
-
- Ana C. G.-C., Noah G., Lestat R. A., Christina H., Jennifer D., Khanh D., et al. (2025). Phase I study of ribociclib (CDK4/6 inhibitor) with spartalizumab (PD-1 inhibitor) with and without fulvestrant in metastatic hormone receptor-positive breast cancer or advanced ovarian cancer. J. Immunother. Cancer 13 (2), e010430. 10.1136/jitc-2024-010430 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

