CXCR3 inhibitors for therapeutic interventions: current status and perspectives
- PMID: 40786052
- PMCID: PMC12331677
- DOI: 10.3389/fphar.2025.1556196
CXCR3 inhibitors for therapeutic interventions: current status and perspectives
Abstract
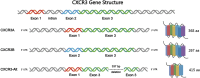
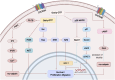
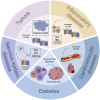
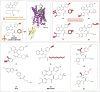
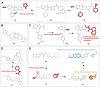
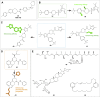
CXC chemokine receptor 3 (CXCR3) is a G protein-coupled chemokine receptor that plays a key role in regulating immune responses and is involved in various pathological processes, particularly in tumor development and inflammatory diseases, making it a novel target for clinical therapy. The expression of CXCR3 and its ligands-CXCL9, CXCL10, CXCL11, CXCL4, and CXCL4L1-is closely associated with the onset and progression of numerous diseases. With a deeper understanding of the mechanisms underlying CXCR3 function, significant progress has been made in the development of small molecule antagonists targeting CXCR3, some of which have entered clinical trials and demonstrated therapeutic potential. This review provides an overview of the structure and signaling pathways of CXCR3, its biological functions in cancer and inflammatory diseases, and highlights the innovative roles of CXCR3 in these diseases. Furthermore, it discusses recent advances in the development of small molecule antagonists, particularly those that have been tested in clinical settings, such as AMG 487 and ACT-777991. These studies provide a scientific foundation for the development of novel CXCR3 antagonists and may offer new directions for future clinical treatments.
Keywords: CXCR3; CXCR3 inhibitors; future clinical treatments; inflammatory diseases,; molecule antagonists,; tumor.
Copyright © 2025 Huo, Jiang, Zhang, Du and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

