Design and synthesis of a chemically diverse, lead-like DNA-encoded library from sequential amide coupling
- PMID: 40786536
- PMCID: PMC12333441
- DOI: 10.1039/d5md00350d
Design and synthesis of a chemically diverse, lead-like DNA-encoded library from sequential amide coupling
Abstract
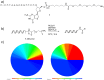
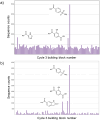
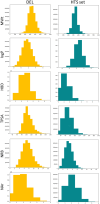
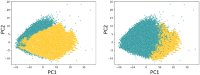
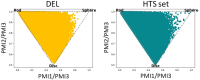
DNA-encoded libraries (DELs) are established as an effective screening strategy to identify protein ligands and offer a cost-effective means of screening large numbers of compounds. However, the synthesis and utilisation of DELs is implemented by relatively few laboratories. Here, we describe the design and synthesis of a medium-sized DEL through simple amide coupling procedures. We provide details of chemistry and enzymatic steps and demonstrate their effectiveness by synthesising 300 thousand and 3 million-member DELs. We demonstrate their integrity through screening against carbonic anhydrase IX and show their chemical diversity through in silico comparison with an established high-throughput screening library. The DELs described can be used as a resource to accelerate hit identification for early-phase drug discovery and are available to the academic community for screening.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Solid-phase DNA-encoded library synthesis: a master builder's instructions.Nat Protoc. 2025 May 22. doi: 10.1038/s41596-025-01190-4. Online ahead of print. Nat Protoc. 2025. PMID: 40404924 Review.
-
High-throughput library transgenesis in Caenorhabditis elegans via Transgenic Arrays Resulting in Diversity of Integrated Sequences (TARDIS).Elife. 2023 Jul 4;12:RP84831. doi: 10.7554/eLife.84831. Elife. 2023. PMID: 37401921 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Abrocitinib, tralokinumab and upadacitinib for treating moderate-to-severe atopic dermatitis.Health Technol Assess. 2024 Jan;28(4):1-113. doi: 10.3310/LEXB9006. Health Technol Assess. 2024. PMID: 38343072 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
References
-
- Halford B. How DNA-Encoded Libraries Are Revolutionizing Drug Discovery. Chem. Eng. News. 2019;95(25):28–33.
-
- Satz A. L. Brunschweiger A. Flanagan M. E. Gloger A. Hansen N. J. V. Kuai L. Kunig V. B. K. Lu X. Madsen D. Marcaurelle L. A. Mulrooney C. O'Donovan G. Sakata S. Scheuermann J. DNA-Encoded Chemical Libraries. Nat. Rev. Methods Primers. 2022;2:3.
LinkOut - more resources
Full Text Sources

