Immune landscape and TAM density in endometrial cancer: implications for immune checkpoint inhibitors efficacy
- PMID: 40786594
- PMCID: PMC12334829
- DOI: 10.1177/17588359251347364
Immune landscape and TAM density in endometrial cancer: implications for immune checkpoint inhibitors efficacy
Abstract
Background: Immune checkpoint inhibitors (ICIs) have shown efficacy in endometrial cancer (EC); however, their efficacy varies according to mismatch repair (MMR) status. Notably, even among patients with MMR-deficient (MMRd) or microsatellite instability-high (MSI-H) tumors, approximately one-third exhibit primary resistance to ICI monotherapy.
Objectives: We aimed to characterize dissimilarities in the tumor immune microenvironment of ICI-treated MMRd/MSI-H versus MMR-proficient (MMRp)/microsatellite stable (MSS) EC, and to identify mechanisms of resistance.
Design: Adults with histologically confirmed advanced or recurrent EC treated with ICIs in 6 French comprehensive cancer centers were included. Patients without available archival formalin-fixed paraffin-embedded primary tumor samples were excluded. Clinical data were collected retrospectively.
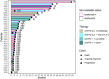
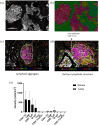
Methods: Patients were classified as ICI-Responders or Non-Responders based on best objective response. A seven-color multi-immunofluorescence staining (CD20, CD4, CD8, FoxP3, CD68, CK, and DAPI) was performed on sections from archival formalin-fixed paraffin-embedded primary tumors. Cell densities and spatial proximity were analyzed using inForm software. T/B lymphoid aggregates (LA) and tertiary lymphoid structures (TLS) were separately quantified. Microsatellite status, presence of LA/TLS, and immune cell densities were correlated to response to treatment.
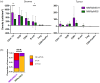
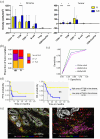
Results: Twenty-one MMRd/MSI-H and 12 MMRp/MSS tumors were analyzed. We observed more MMRd/MSI-H tumors with LA/TLS compared to MMRp/MSS cases: 81% versus 17%, p ⩽ 0.001. There were more CD8+ T effector cells in the vicinity of B cells in MMRd/MSI-H tumors compared to MMRp/MSS tumors (1.26 (0-3.40) vs 0.49 (0-1.86), p = 0.017), suggesting cooperation between CD8+ T cells and B cells in MMRd/MSI-H tumors. No differences were shown in terms of the presence of LA/TLS and the subsequent response to ICI in EC (p = 0.400). Using a multivariate logistic regression model, we found that a low density of CD68+ tumor-associated macrophages (TAMs) in the stroma, was associated with response to ICI in EC (odds ratio = 11.67, 95% CI (1.69-237.45), p = 0.033) and showed good accuracy in predicting response to ICI in the whole cohort (AUC = 0.75, 95% CI (0.59-0.91)).
Conclusion: We characterize the immune landscape in EC patients treated with ICIs. Distinct immune infiltrate patterns were observed in MMRd/MSI-H and MMRp/MSS tumors. A significant negative association between TAM density and ICI response was shown.
Keywords: endometrial cancer; immune checkpoint inhibitors; macrophages.
Plain language summary
Exploring immune cells and tumor macrophages in endometrial cancer: insights for immunotherapy success Immune checkpoint inhibitors (ICIs) are promising treatments for endometrial cancer (EC), particularly in patients with specific genetic profiles, such as mismatch repair-deficient or microsatellite instability-high (MMRd/MSI-H) tumors. However, these treatments do not work for everyone, and many patients with MMRd/MSI-H tumors show resistance to ICIs. This study analyzed differences in the immune environment of EC tumors with and without these genetic markers to understand why some patients respond better to ICIs. Tumor samples from 33 patients treated with ICIs were studied using advanced staining techniques to visualize immune cells. Researchers also measured the densities of various immune cells, including T cells, B cells, and tumor-associated macrophages (TAMs), and examined their interactions within the tumors. The study found that MMRd/MSI-H tumors had more immune structures, such as lymphoid aggregates and tertiary lymphoid structures, compared to other types of EC. These tumors also showed more collaboration between two types of immune cells: CD8+ T cells (which attack cancer cells) and B cells. However, the presence of these structures did not predict whether patients would respond to ICI treatment. Interestingly, the researchers discovered that a lower number of TAMs, which can suppress the immune response, was strongly linked to better responses to ICIs. This finding suggests that TAM density could serve as a potential biomarker to predict which patients might benefit most from this therapy. Overall, this study highlights differences in immune environments between EC tumor types and identifies TAMs as a potential target to improve ICI efficacy. This research may help refine treatment strategies and improve outcomes for patients with endometrial cancer.
© The Author(s), 2025.
Conflict of interest statement
A.M. received honoraria from GSK. J.-S.F. received honoraria from AstraZeneca, Daïchi, Pfizer, Lilly, Novartis, Gsk, MSD, Esai, and Seagen. I.R.-C. received research grants from MSD, Roche, BMS, GSK, Novartis, AstraZeneca, and Merck Sereno; honoraria from Abbvie, Agenus, Advaxis, BMS, PharmaMar, Genmab, Pfizer, AstraZeneca, Roche/Genentech, GSK, MSD, Deciphera, Mersena, Merck Sereno, Novartis, Amgen, Tesaro and Clovis; Springworks, Adaptimmune, Immunogen, Seagen, Novocure, Daichi Sankyo; and travel support from Roche, AstraZeneca, and GSK. R.S. received research grants from AstraZeneca, honoraria from AstraZeneca, GSK, Seagen, EISAI, Novartis, Clovis Oncology, and nonfinancial support from MSD, GSK, and Novartis. O.L.S. received research grants from AstraZeneca and honoraria from GSK, MSD, and Clovis Oncology.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6): 394–424. - PubMed
-
- Mirza MR, Chase DM, Slomovitz BM, et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med 2023; 388(23): 2145–2158. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

