Nanobodies in animal infectious disease control: diagnosis and therapy
- PMID: 40786610
- PMCID: PMC12331703
- DOI: 10.3389/fcimb.2025.1640352
Nanobodies in animal infectious disease control: diagnosis and therapy
Abstract
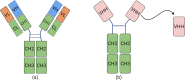
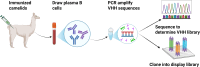
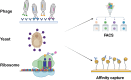
Animal infectious diseases threaten livestock productivity, public health, and food security. Traditional monoclonal antibodies (mAbs) face limitations in diagnostics and therapy due to their large size, instability, and high cost. Nanobodies (Nbs), derived from camelid heavy-chain antibodies, offer superior properties-small size (~15 kDa), high stability, deep tissue penetration, and cost-effective production. Nbs feature extended CDR3 loops, enabling access to cryptic epitopes, and exhibit exceptional thermal/pH stability. They are generated by immunizing camelids, cloning VHH genes, and screening via phage/yeast display. High-throughput methods (ELISA, flow cytometry) allow rapid isolation of high-affinity Nbs. Compared to mAbs, Nbs are economically produced in prokaryotic systems and engineered into multivalent or Fc-fused formats for enhanced efficacy. In diagnostics, Nbs enable sensitive, low-cost detection of pathogens like PRRSV, ASFV, and avian influenza. Nb-based competitive ELISAs and lateral flow assays improve field surveillance. Therapeutically, Nbs neutralize pathogens by targeting viral proteins (e.g., blocking PRRSV-CD163 entry) or bacterial toxins (e.g., Staphylococcus enterotoxins). Nb-Fc fusions degrade ASFV proteins via TRIM-away, while intracellular Nbs disrupt Mycobacterium ESAT-6 or Toxoplasma actin dynamics. Challenges remain in Nb affinity optimization, intracellular delivery, and in vivo half-life. Solutions include fusion with cell-penetrating peptides or viral vectors (e.g., adenoviruses). Reducing cross-species immunogenicity and scaling production are critical for broader adoption. With advances in protein engineering, Nbs hold transformative potential for preventing, diagnosing, and treating animal diseases, offering scalable solutions for global health and food security.
Keywords: ASFV; PRRSV; animal infectious diseases; diagnostics; nanobodies; phage display; therapeutics.
Copyright © 2025 Wang, Tong and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Applications of Nanobodies in Biological Imaging.Cancer Biother Radiopharm. 2025 Aug;40(6):365-376. doi: 10.1089/cbr.2025.0005. Epub 2025 Apr 24. Cancer Biother Radiopharm. 2025. PMID: 40274307 Review.
-
Research Progress on the Application of Neutralizing Nanobodies in the Prevention and Treatment of Viral Infections.Microorganisms. 2025 Jun 11;13(6):1352. doi: 10.3390/microorganisms13061352. Microorganisms. 2025. PMID: 40572239 Free PMC article. Review.
-
A Novel Nanobody Targeting Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Receptor-Binding Domain Has Potent Cross-Neutralizing Activity and Protective Efficacy against MERS-CoV.J Virol. 2018 Aug 29;92(18):e00837-18. doi: 10.1128/JVI.00837-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29950421 Free PMC article.
-
A phage-displayed nanobody-based competitive immunoassay for the detection of African swine fever virus antibodies.Virol J. 2025 Jun 5;22(1):182. doi: 10.1186/s12985-025-02781-z. Virol J. 2025. PMID: 40474190 Free PMC article.
-
Characterization of Nanobody Binding to Distinct Regions of the SARS-CoV-2 Spike Protein by Flow Virometry.Viruses. 2025 Apr 15;17(4):571. doi: 10.3390/v17040571. Viruses. 2025. PMID: 40285013 Free PMC article.
References
-
- Bates T. A., Trank-Greene M., Nguyenla X., Anastas A., Gurmessa S. K., Merutka I. R., et al. (2024). ESAT-6 undergoes self-association at phagosomal pH and an ESAT-6-specific nanobody restricts M. tuberculosis growth in macrophages. Elife 12:RP91930. doi: 10.7554/eLife.91930, PMID: - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

