Component characterization of Smilax glabra Roxb., and its inhibitory activity against Helicobacter pylori through targeted suppression of its secreted urease
- PMID: 40786612
- PMCID: PMC12331786
- DOI: 10.3389/fcimb.2025.1617330
Component characterization of Smilax glabra Roxb., and its inhibitory activity against Helicobacter pylori through targeted suppression of its secreted urease
Abstract
Background: Smilax glabra Roxb. (SGR), known as "tufuling" in China, is a medical and edible plant, which has anti-inflammatory, antibacterial and antineoplastic activity. SGR is extensively utilized in the remedy of gastroenteric disorders associated with H. pylori infection. However, the precise mechanism underlying the anti-H. pylori function of SGR remains to be elucidated.
Aim: The inhibitory impact of SGR on the growth of H. pylori was examined. Subsequently, SGR against H. pylori urease (HPU) and jack bean urease (JBU) was investigated to illuminate the inhibitory effects, kinetic types, sites of inhibition, and potential mechanisms of action.
Methods: UPLC-ESI-MS/MS was applied to identify the components of SGR. The anti-H. pylori effect of SGR was conducted by agar dilution method. The enzyme inhibitory activities of SGR and its primary constituents were assessed through a modified spectrophotometric Berthelot (phenol-hypochlorite) assay. The kinetics of urease inhibition were analyzed using Lineweaver-Burk plots. To explore the underlying mechanisms, sulfhydryl group reagents and Ni2+ binding depressors were employed. Additionally, molecular docking simulations were conducted to examine the binding interactions between the main compounds of SGR and urease.
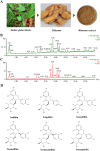
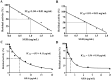
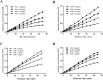
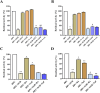
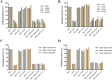
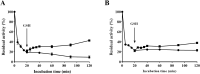
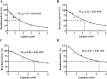
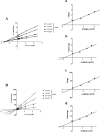
Results: A total of 34 compounds including astilbin, engeletin, isoengeletin, neoastilbin, isoastilbin and neoisoastilbin are identified in SGR. SGR was observed to inhibit the growth of three H. pylori strains (ATCC 43504, NCTC 26695, and ICDC 111001) with minimum inhibitory concentration (MIC) values spanning a range of 0.5 to 1.5 mg/mL. Moreover, SGR exerted a significant inhibitory effect on HPU and JBU, with IC50 values of 1.04 ± 0.01 mg/mL and 1.01 ± 0.01 mg/mL, separately. Enzyme kinetics analysis showed that SGR was a slow binding, non-competitive depressor to HPU, and a slow binding, mixed depressor to JBU. In-depth mechanism exploration showed that thiol compounds had better protective effect on HPU or JBU than inorganic substances, implying that the active site of SGR repressing urease may be the sulfhydryl group. Furthermore, glutathione reactivated SGR-inhibited urease, demonstrating that the inhibition was reversible. Additionally, astilbin and engeletin exhibited a certain inhibitory role towards urease activity, with astilbin inhibiting urease more than three times as strongly as engelitin. Enzyme kinetics analysis established that the inhibitory role of astilbin on enzymes was consistent with that of SGR. Molecular docking study indicated that astilbin and engeletin interacts with sulfhydryl groups at the active site of urease.
Conclusion: These results indicated that SGR could prominently inhibit H. pylori growth through targeted suppression of its secreted urease. This investigation provides substantial experimental evidence supporting the consideration of SGR as a safe and promising natural treatment for H. pylori-associated gastrointestinal diseases.
Keywords: Helicobacter pylori; Smilax glabra Roxb.; astilbin; molecular docking; thiol; urease.
Copyright © 2025 Tang, Yang, Wen, Zhou, Tang, He, Lu and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Insight into the inhibitory effects of Zanthoxylum nitidum against Helicobacter pylori urease and jack bean urease: Kinetics and mechanism.J Ethnopharmacol. 2020 Mar 1;249:112419. doi: 10.1016/j.jep.2019.112419. Epub 2019 Nov 20. J Ethnopharmacol. 2020. PMID: 31759110
-
Phytochemical analysis of dried ginger extract and its inhibitory effect and mechanism on Helicobacter pylori and associated ureases.Food Funct. 2025 Feb 3;16(3):1100-1115. doi: 10.1039/d4fo04991h. Food Funct. 2025. PMID: 39831446
-
Inhibition of Helicobacter pylori and Its Associated Urease by Palmatine: Investigation on the Potential Mechanism.PLoS One. 2017 Jan 3;12(1):e0168944. doi: 10.1371/journal.pone.0168944. eCollection 2017. PLoS One. 2017. PMID: 28045966 Free PMC article.
-
Sequential versus standard triple first-line therapy for Helicobacter pylori eradication.Cochrane Database Syst Rev. 2016 Jun 28;2016(6):CD009034. doi: 10.1002/14651858.CD009034.pub2. Cochrane Database Syst Rev. 2016. PMID: 27351542 Free PMC article.
-
Non-invasive diagnostic tests for Helicobacter pylori infection.Cochrane Database Syst Rev. 2018 Mar 15;3(3):CD012080. doi: 10.1002/14651858.CD012080.pub2. Cochrane Database Syst Rev. 2018. PMID: 29543326 Free PMC article.
References
-
- Abaidullah M., La S., Liu M., Liu B., Cui Y., Wang Z., et al. (2023). Polysaccharide from Smilax glabra Roxb mitigates intestinal mucosal damage by therapeutically restoring the interactions between gut microbiota and innate immune functions. Nutrients 15, 4102. doi: 10.3390/nu15194102, PMID: - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

