Triplet systemic therapy for hormone-sensitive prostate cancer: a critical review with a multidisciplinary approach
- PMID: 40787089
- PMCID: PMC12331671
- DOI: 10.3389/or.2025.1599292
Triplet systemic therapy for hormone-sensitive prostate cancer: a critical review with a multidisciplinary approach
Abstract
This article aims to critically evaluate the evidence for triplet therapy consisting of androgen deprivation therapy (ADT), docetaxel and a second-generation androgen receptor pathway inhibitor ([ARPI]; abiraterone, enzalutamide, darolutamide or apalutamide) in patients with metastatic hormone-sensitive prostate cancer (mHSPC), and what this evidence reveals regarding the use of these treatments in clinical practice. A search of PubMed, Medline, Embase, Cochrane, Scopus and Web of Science was conducted in April 2024 to identify relevant prospective and retrospective observational trials, randomized controlled trials (RCTs) and meta-analyses. The search identified 52 relevant articles: six full articles and 31 abstracts based on three RCTs, one observational study and 14 meta-analyses. Abiraterone- or darolutamide-containing triplet therapy was significantly better than ADT + docetaxel for improving overall survival in all study populations, particularly subgroups with high-volume and/or synchronous disease. The tolerability of ADT + docetaxel and triplet therapy were similar with most adverse events related to docetaxel. There were no data comparing triplet therapy with ADT + ARPI doublet therapy. Triplet therapy appears to be the most effective first-line regimen for men with mHSPC, good performance status and high-volume and synchronous metastases. Darolutamide-based triplet therapy may also be of benefit in other patients with high- or low-risk disease. Careful consideration of the risks and benefits are required to determine which patients can be spared from receiving docetaxel and rather be treated with alternative regimens.
Keywords: androgen deprivation therapy; androgen receptor-targeted therapy; docetaxel; hormone-sensitive prostate cancer; metastatic prostate cancer.
Copyright © 2025 Zapatero, Alonso-Gordoa, Rodríguez Antolín, Couñago, Sanmamed, Domínguez Esteban, López Valcárcel, Manneh, Borque-Fernando, Sala González and Maroto.
Conflict of interest statement
AZ has received speaker honoraria from Astellas-Pharma; travel expenses from Ipsen, Recordati, and Janssen; and research grants from Janssen. TA-G has received fees for speaker, consultancy, research and other non-financial support from IPSEN, Eli Lilly, Adacap, Pfizer, EISAI, Bayer, Johnson & Johnson, Astellas-Pharma, Roche and MSD. AR has received honoraria for his participation in training and consultancy sessions from Astellas-Pharma, AstraZeneca, Novartis, Bayer and Janssen. FC has received honoraria for participation in expert committees and conferences from Janssen, Astellas, IPSEN, Recordati, Boston Scientific, AstraZeneca and Bayer. NS has served on an advisory board for Astellas-Pharma, and received speaker honoraria from Astellas-Pharma, Janssen, and Bayer, and travel expenses from Ipsen and Recordati. MD has received honoraria for participation in expert committees and conferences from Janssen, Astellas, IPSEN, Bristol Myers Squibb, AAA, Boston Scientific, Intuitive, AstraZeneca and Bayer. ML has received speaker honoraria from Astellas-Pharma, Janssen, and Bayer. RM has served as a speaker, advisory board member and clinical researcher for Astellas, Amgen, Jannsen, Bayer, Pfizer, Novartis, BMS, MSD, Eli Lilly, Roche, Ipsen, Merck Serono and Adium. ÁB-F has participated in training sessions and has received consultancy fees from Asofarma, Astellas-Pharma, AstraZeneca, Bayer, GP Pharm, HealthMDx, Ipsen, Janssen, Lacer, MSD, Pharmalink and Recordati. NS has received research grants, honoraria, and other non-financial support from IPSEN, Pfizer, Bayer, BMS and MSD. PM has received speaker and consultancy fees, and other support such as logistical assistance from Astellas, Janssen, Bayer, MSD and Novartis.
Figures
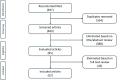
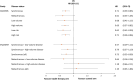
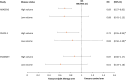
Similar articles
-
Triplet or Doublet Therapy in Metastatic Hormone-sensitive Prostate Cancer Patients: A Systematic Review and Network Meta-analysis.Eur Urol Focus. 2023 Jan;9(1):96-105. doi: 10.1016/j.euf.2022.08.007. Epub 2022 Sep 1. Eur Urol Focus. 2023. PMID: 36058809
-
Androgen Receptor Signaling Inhibitors in Addition to Docetaxel with Androgen Deprivation Therapy for Metastatic Hormone-sensitive Prostate Cancer: A Systematic Review and Meta-analysis.Eur Urol. 2022 Dec;82(6):584-598. doi: 10.1016/j.eururo.2022.08.002. Epub 2022 Aug 19. Eur Urol. 2022. PMID: 35995644
-
Triplet or Doublet Therapy in Metastatic Hormone-sensitive Prostate Cancer: Updated Network Meta-analysis Stratified by Disease Volume.Eur Urol Focus. 2023 Sep;9(5):838-842. doi: 10.1016/j.euf.2023.03.024. Epub 2023 Apr 11. Eur Urol Focus. 2023. PMID: 37055323
-
Treatments for Metastatic Hormone-sensitive Prostate Cancer: Systematic Review, Network Meta-analysis, and Benefit-harm assessment.Eur Urol Oncol. 2022 Dec;5(6):605-616. doi: 10.1016/j.euo.2022.04.007. Epub 2022 May 20. Eur Urol Oncol. 2022. PMID: 35599144
-
Impact of disease volume on survival efficacy of triplet therapy for metastatic hormone-sensitive prostate cancer: a systematic review, meta-analysis, and network meta-analysis.Int J Clin Oncol. 2024 Jun;29(6):716-725. doi: 10.1007/s10147-024-02485-4. Epub 2024 Apr 6. Int J Clin Oncol. 2024. PMID: 38582807 Free PMC article.
References
-
- International Agency for Research on Cancer. Cancer today data: prostate cancer. International Agency for Research on Cancer; (2022). Available online at: https://gco.iarc.fr/today/en/dataviz/tables?mode=population&cancers=27 [Accessed February 28, 2024].
-
- International Agency for Research on Cancer. Cancer tomorrow data: prostate cancer. Int Agency Res Cancer (2022). Available online at: https://gco.iarc.fr/tomorrow/en/dataviz/tables?cancers=27 [Accessed February 28, 2024].
-
- International Agency for Research on Cancer. Global cancer observatory fact sheet. Prostate: Int Agency Res Cancer (2022). Available online at: https://gco.iarc.who.int/media/globocan/factsheets/cancers/27-prostate-f... [Accessed February 28, 2024].
-
- de Velasco Oria de Rueda G, Plata Bello AC, Landeira M, Mateo M, Anguita P, Pranzo A, et al. Incidence, prevalence, and treatment patterns in metastatic hormone-sensitive prostate cancer in Spain: ECHOS study. Actas Urológicas Españolas (English Edition) (2022) 46(9):557–64. 10.1016/j.acuroe.2022.02.009 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

