Mechanistic and structural characterization of an iridium-containing cytochrome reveals kinetically relevant cofactor dynamics
- PMID: 40787229
- PMCID: PMC12333895
- DOI: 10.1038/s41929-022-00899-9
Mechanistic and structural characterization of an iridium-containing cytochrome reveals kinetically relevant cofactor dynamics
Abstract
Artificial metalloenzymes (ArMs), which contain non-native, typically synthetic, metal cofactors, are a flourishing class of biocatalyst for unnatural reactions. Although the number of these reactions is rapidly increasing, multi-faceted mechanistic studies of ArMs comprising structural, kinetic, computational and cofactor binding data to reveal detailed mechanistic information on the effects of the protein scaffold on the structure and reactivity of ArMs are more limited. Here we report the structure of an unnatural P450 analogue using X-ray diffraction. We also report the kinetic analysis of its reaction, catalyst activation during an induction period, and the origins of the stereoselectivity for the cyclopropanation of a terpene catalysed by the iridium-containing P450 variant (Ir(Me)-CYP119). Our data reveal a mechanism initiated by the flip of the cofactor from an inactive to an active conformation. This change in conformation is followed by thousands of turnovers occurring by rate-determining formation of an iridium-carbene intermediate, thereby highlighting the influence of cofactor dynamics within a single active site on an ArM-catalysed reaction.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures



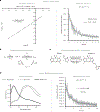


References
Grants and funding
LinkOut - more resources
Full Text Sources
