Imaging Findings in Neurogenic Ptosis
- PMID: 40787295
- PMCID: PMC12328912
- DOI: 10.3348/jksr.2024.0107
Imaging Findings in Neurogenic Ptosis
Abstract
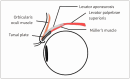
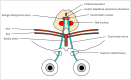
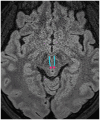
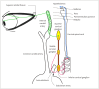
Neurogenic ptosis refers to upper eyelid drooping resulting from disrupted innervation of the eyelid retractor muscles. The differential diagnoses of neurogenic ptosis include oculomotor nerve palsy, Horner's syndrome, and neuromuscular junction disorders. Oculomotor nerve palsy and Horner's syndrome result from damage to the oculomotor and oculosympathetic pathways, respectively. The oculomotor nerve pathway has four segments: intraaxial, subarachnoid, cavernous, and orbital. The oculosympathetic pathway consists of three orders of neurons extending from the base of the skull to the upper chest. Several pathologic conditions can affect these neural pathways and cause neurogenic ptosis owing to the long course of the involved nerves. Therefore, neurogenic ptosis is usually associated with unique clinical features based on the anatomical location of the pathology. This pictorial essay provides a deeper understanding of the neural pathways and different diseases that cause neurogenic ptosis, which can help in determining the location of the pathology.
신경성 안검하수는 눈꺼풀을 들어 올리는 근육을 지배하는 신경에 문제가 생겨 눈꺼풀이 처지는 것을 말한다. 신경성 안검하수의 감별진단에는 안구운동신경마비, 호너증후군, 중증근육무력증을 포함한 신경근 접합부 장애 등이 있다. 특히 안구운동신경마비와 호너증후군은 각각 안구운동신경 경로와 안교감신경 경로의 손상에 의해 발생한다. 안구운동신경 경로는 축내분절, 지주막하분절, 해면분절, 안와분절의 4개 분절로 구성된다. 안교감신경 경로는 1차, 2차, 3차 뉴런으로 구성되며 두개골 기저부에서부터 가슴 상부에 걸쳐 있다. 이 신경들은 긴 경로로 이루어져 있기 때문에 다양한 질환들이 이 신경 경로에 영향을 미칠 수 있으며, 보통 해부학적 위치에 따라 독특한 임상적 특징들과 관련이 있다. 이 임상화보의 목적은 신경성 안검하수을 일으키는 신경 경로와 질병의 종류를 이해하고, 신경성 안검하수의 원인이 되는 위치를 유추할 수 있도록 하는 것이다.
Keywords: Anatomy; Horner’s Syndrome; Imaging; Oculomotor Nerve Palsy; Ptosis.
Copyrights © 2025 The Korean Society of Radiology.
Conflict of interest statement
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Figures
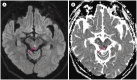
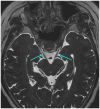
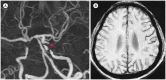
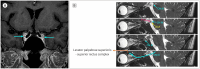
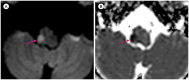
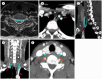
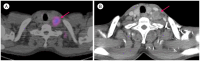
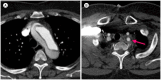
Similar articles
-
Ptosis Correction.2023 Jul 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 Jul 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30969650 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
A rare presentation of Horner's syndrome following cervical epidural steroid injection.Interv Pain Med. 2025 Jul 29;4(3):100615. doi: 10.1016/j.inpm.2025.100615. eCollection 2025 Sep. Interv Pain Med. 2025. PMID: 40761434 Free PMC article.
-
Uncommon Non-MS Demyelinating Disorders of the Central Nervous System.Curr Neurol Neurosci Rep. 2025 Jul 1;25(1):45. doi: 10.1007/s11910-025-01432-8. Curr Neurol Neurosci Rep. 2025. PMID: 40591029 Review.
-
Interventions for eye movement disorders due to acquired brain injury.Cochrane Database Syst Rev. 2018 Mar 5;3(3):CD011290. doi: 10.1002/14651858.CD011290.pub2. Cochrane Database Syst Rev. 2018. PMID: 29505103 Free PMC article.
References
-
- Cahill KV, Burns JA, Weber PA. The effect of blepharoptosis on the field of vision. Ophthalmic Plast Reconstr Surg. 1987;3:121–125. - PubMed
-
- Federici TJ, Meyer DR, Lininger LL. Correlation of the vision-related functional impairment associated with blepharoptosis and the impact of blepharoptosis surgery. Ophthalmology. 1999;106:1705–1712. - PubMed
-
- Finsterer J. Ptosis: causes, presentation, and management. Aesthetic Plast Surg. 2003;27:193–204. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

