A Bright Spiropyran-Based Zinc Sensor for Live-Cell Imaging
- PMID: 40787398
- PMCID: PMC12332670
- DOI: 10.1021/acsomega.5c04186
A Bright Spiropyran-Based Zinc Sensor for Live-Cell Imaging
Abstract
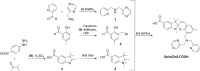
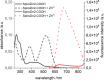
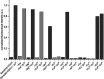
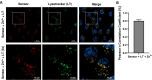
Pools of labile bound zinc ions are essential for signal transduction in the human body. At the cellular level, such pools occur in the cytosol, discrete organelles, and secretory vesicles. These zinc-containing vesicles are found in distinct regions of the central nervous system, modulating calcium ion channels that play an essential role in olfaction, audition, and somatosensory perception. Dysregulation of these receptors is associated with a number of neurodegenerative diseases. To understand the underlying mechanisms at the molecular level, zinc fluorescence sensors are versatile tools. In this report, a new member of the spiropyran-based sensor family SpiroZin, which has proven useful for the investigation of zinc in living cells, is presented: SpiroZin2-COOH. This sensor can be synthesized in a 5-step synthesis and shows superior zinc-sensing properties in cuvette as well as live cell studies. The quantum yield is approximately seven times higher than that of the parent zinc sensor, which also results in an approximately 6-fold higher brightness and a turn-on of 30 at pH 7 in cuvette studies. Another advantage is a significant red-shift of 30 nm in comparison to the parent sensor SpiroZin2. Other basic properties of the SpiroZin family are retained, as revealed by a similar binding constant and negligible pH dependence in zinc sensing. Similar to other members of the SpiroZin family, SpiroZin2-COOH images intracellular zinc pools in living cells. Lysotracker costaining reveals lysosomal localization of SpiroZin2-COOH. The turn-on is determined to be 14.6, which is the highest turn-on within the SpiroZin family reported so far in live-cell studies.
© 2025 The Authors. Published by American Chemical Society.
Figures


References
-
- Vallee B. L., Falchuk K. H.. Zinc uptake and distribution in X. laevis oocytes and embryos. Physiol. Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. - DOI - PubMed
- Kelleher S. L., McCormick N. H., Velasquez V., Lopez V.. Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv. Nutr. 2011;2:101–111. doi: 10.3945/an.110.000232. - DOI - PMC - PubMed
-
- Kambe T., Tsuji T., Hashimoto A., Itsumura N.. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015;95:749–784. doi: 10.1152/physrev.00035.2014. - DOI - PubMed
- Krężel A., Maret W.. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016;611:3–19. doi: 10.1016/j.abb.2016.04.010. - DOI - PMC - PubMed
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”.Int. J. Mol. Sci. 2017, 18, 11. - PMC - PubMed
- Maret W., Krężel A.. Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease. Mol. Med. 2007;13:371–375. doi: 10.2119/2007-00036.Maret. - DOI - PMC - PubMed
- Chang, C. J. ; Lippard, S. J. . Zinc Metalloneurochemistry: physiology, pathology, and probes. In Metal Ions in Life Sciences 1: neurodegenerative Diseases and Metal Ions, 1st ed.; Sigel, A. ; Sigel, H. ; Sigel, R. K. O. , Eds.; John Wiley & Sons: Chichester, 2006; pp. 281–320.
LinkOut - more resources
Full Text Sources
