Fasting Induces Gene Expression of Insulin-like Growth Factor-binding Proteins in Skeletal Muscles of Chicks
- PMID: 40787579
- PMCID: PMC12326139
- DOI: 10.2141/jpsa.2025022
Fasting Induces Gene Expression of Insulin-like Growth Factor-binding Proteins in Skeletal Muscles of Chicks
Abstract
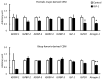
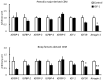
In mammals, evidence suggests that insulin-like growth factor-binding proteins (IGFBPs) affect skeletal muscle growth in an autocrine and paracrine manner. In the present study, fasting induced significant transcriptional changes in IGFBP genes in the skeletal muscles of layer and broiler chickens. Twelve hours of fasting significantly increased mRNA levels of IGFBP-1 in the biceps femoris (BF; largest skeletal muscle in the thigh) of both chicken types. mRNA levels of IGFBP-2 in both the pectoralis major (PM; breast muscle) and the BF significantly increased in layer chicks and tended to increase in broiler chicks. Fasting significantly decreased mRNA levels of IGFBP-3 in the BF and PM of both chicken type. mRNA levels of IGFBP-4 and -5 differed responses in the PM and BF of layer and broiler chicks. mRNA levels of most IGFBP genes were not affected by insulin-like growth factor-1 (IGF-1) in chicken embryonic myotubes, suggesting that skeletal muscle IGFBPs were transcriptionally regulated in an IGF-1-independent manner. Overall, these findings suggested that IGFBP-1, -2, and -3, which were expressed in skeletal muscles, played conserved roles in layer and broiler chicks.
Keywords: chick; chicken; feeding; starvation.
2025 Japan Poultry Science Association.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflict of interests.
Figures


Similar articles
-
Insulin-like growth factor binding protein-6 modulates proliferative antagonism in response to progesterone in breast cancer.Front Endocrinol (Lausanne). 2024 Dec 4;15:1450648. doi: 10.3389/fendo.2024.1450648. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39698031 Free PMC article.
-
Cord blood levels of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 correlate with perinatal brain development in fetal congenital heart disease.Ultrasound Obstet Gynecol. 2025 Aug;66(2):200-209. doi: 10.1002/uog.29271. Epub 2025 Jun 28. Ultrasound Obstet Gynecol. 2025. PMID: 40580544 Free PMC article.
-
Production of insulin-like growth factor binding proteins by human ovarian carcinoma cells.J Cancer Res Clin Oncol. 1994;120(3):137-42. doi: 10.1007/BF01202191. J Cancer Res Clin Oncol. 1994. PMID: 7505272 Free PMC article.
-
Insulin-like growth factor binding protein-1 in PCOS: a systematic review and meta-analysis.Hum Reprod Update. 2011 Jan-Feb;17(1):4-16. doi: 10.1093/humupd/dmq027. Epub 2010 Jul 15. Hum Reprod Update. 2011. PMID: 20634211
-
Does milk intake promote prostate cancer initiation or progression via effects on insulin-like growth factors (IGFs)? A systematic review and meta-analysis.Cancer Causes Control. 2017 Jun;28(6):497-528. doi: 10.1007/s10552-017-0883-1. Epub 2017 Mar 30. Cancer Causes Control. 2017. PMID: 28361446 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

