Translocation of penetratin-like peptides involving calcium-dependent interactions between glycosaminoglycans and phosphocholine headgroups of the membrane lipid bilayer
- PMID: 40787615
- PMCID: PMC12332767
- DOI: 10.1039/d5cb00099h
Translocation of penetratin-like peptides involving calcium-dependent interactions between glycosaminoglycans and phosphocholine headgroups of the membrane lipid bilayer
Abstract
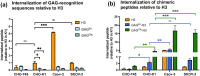
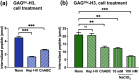
Cell-penetrating peptides (CPPs) can internalize ubiquitously in cells. To explore the specific targeting issue of CPPs, we used glycosaminoglycan (GAG)-binding peptides previously identified in Otx2 and En2 homeoproteins (HPs). The Otx2 sequence preferentially recognizes highly sulfated chondroitin (CS) and the En2 one, heparan sulfates (HS) GAGs. The two HPs internalize in specific cells thanks to their GAG-targeting sequence. We studied the capacity of chimeric peptides containing a GAG-targeting and a penetratin-like sequences to enter into various cell lines known to express different levels and types of GAGs. Since GAGs are found at the vicinity the membrane lipid bilayer, we also analyzed the putative binary and ternary interactions between heparin (HI), (4S,6S)-CS (CS-E), zwitterionic phosphocholine (PC) model membranes and those chimeric peptides. Altogether, our results demonstrate the existence of Ca2+-dependent interactions between GAGs and PC lipid bilayers, the major phospholipid headgroup found in animal cell plasma membrane. In addition, the interaction of CS-E (but not HI), with PC favors the binding of the chimeric CS-E-recognition motif-penetratin-like peptide and its subsequent crossing of the lipid membrane to access directly to the cytosol of cells. Altogether, this study brings further understanding of translocation mechanism of CPPs, which requires specific GAGs at the cell-surface. It also shed light on the role of GAGs in the cell transfer specificity and paracrine activity of HPs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Basic patches on the E2 glycoprotein of eastern equine encephalitis virus influence viral vascular clearance and dissemination in mice.J Virol. 2025 Jun 17;99(6):e0060225. doi: 10.1128/jvi.00602-25. Epub 2025 May 19. J Virol. 2025. PMID: 40387358 Free PMC article.
-
The quantity, quality and findings of network meta-analyses evaluating the effectiveness of GLP-1 RAs for weight loss: a scoping review.Health Technol Assess. 2025 Jun 25:1-73. doi: 10.3310/SKHT8119. Online ahead of print. Health Technol Assess. 2025. PMID: 40580049 Free PMC article.
-
[Research progress of peptide recognition-guided strategies for exosome isolation and enrichment].Se Pu. 2025 May;43(5):446-454. doi: 10.3724/SP.J.1123.2024.10015. Se Pu. 2025. PMID: 40331609 Free PMC article. Review. Chinese.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

