Mpox: disease manifestations and therapeutic development
- PMID: 40787989
- PMCID: PMC12459235
- DOI: 10.1128/jvi.00152-25
Mpox: disease manifestations and therapeutic development
Abstract
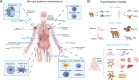
Mpox, caused by monkeypox virus (MPXV) infection, has emerged as a significant global health threat. The World Health Organization (WHO) has twice declared a Public Health Emergency of International Concern for mpox: first for the 2022-2023 global outbreak and subsequently for concurrent outbreaks in Africa. Beyond MPXV, other members of the Orthopoxvirus genus also pose growing risks of zoonotic spillover, with the potential to jump from animal reservoirs to humans. Clinically, mpox is distinguished from other Orthopoxvirus infections by its propensity to cause severe systemic manifestations alongside localized skin lesions, disproportionately affecting vulnerable groups such as children, pregnant women, and immunocompromised individuals. Although vaccines are available, effective therapeutics are equally essential in combating the mpox crisis. Current antiviral agents, including tecovirimat and brincidofovir, have demonstrated uncertain or disappointing efficacy in preclinical and clinical studies, underscoring the urgent need for further therapeutic development. This review provides a concise synthesis of recent advances in understanding mpox epidemiology and clinical features and offers an in-depth discussion of the current status and future directions in therapeutic development. We highlight the importance of innovative experimental models that can authentically replicate mpox disease manifestations and serve as robust platforms for therapeutic testing. Advancing these research efforts is critical for responding to the ongoing mpox emergency and for sustaining preparedness against future poxvirus epidemics.
Keywords: clinical feature; experimental model; monkeypox virus; therapeutic development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Li P, Li J, Ayada I, Avan A, Zheng Q, Peppelenbosch MP, de Vries AC, Pan Q. 2023. Clinical Features, antiviral treatment, and patient outcomes: a systematic review and comparative analysis of the previous and the 2022 Mpox outbreaks. J Infect Dis 228:391–401. doi: 10.1093/infdis/jiad034 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

