Hepatic ENTPD5 Is Critical for Maintaining Metabolic Homeostasis and Promoting Brown Adipose Tissue Thermogenesis
- PMID: 40788055
- PMCID: PMC12561356
- DOI: 10.1002/advs.202503603
Hepatic ENTPD5 Is Critical for Maintaining Metabolic Homeostasis and Promoting Brown Adipose Tissue Thermogenesis
Abstract
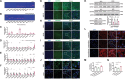
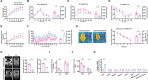
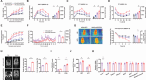
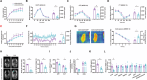
Although hepatocyte-released adenosine triphosphate (ATP) plays important roles in maintaining metabolic homeostasis, how its hydrolyzation outside hepatocytes impacts on glucolipid metabolism remains unclear. The authors aim to identify the enzyme(s) that hydrolyzes hepatocyte-secreted ATP to regulate metabolic homeostasis. All known ATP-hydrolyzing enzymes are expressed with the highest expression of ectonucleoside triphosphate diphosphohydrolase 5 (ENTPD5) in hepatocytes. ENTPD5 expression is reduced in steatotic mouse and human livers. Hepatic ENTPD5 overexpression ameliorates the deregulated glucolipid metabolism and obesity with increased brown adipose tissue (BAT) thermogenesis, while hepatic ENTPD5 silencing exerted the opposite effects in obese mice. Mechanistically, ENTPD5 hydrolyzes extracellular ATP to ADP, which activates purinergic receptor, P2Y12, to inhibit gluconeogenesis and lipid deposition, and repress adrenomedullin (ADM) expression. Hepatic ENTPD5 repression promotes ADM expression and release to inhibit uncoupling protein 1 (UCP1) expression and thermogenesis in BAT. Hepatic ADM expression is increased in NAFLD patients. Serum ADM level is correlated positively with Body mass index in overweighted or obese humans. Hepatic ADM silencing promotes UCP1 expression and thermogenesis in BAT of obese mice. Overall, ENTPD5-mediated hydrolysis of extracellular ATP to ADP of hepatocytes is critical for maintaining hepatic glucose/lipid metabolism and promoting BAT thermogenesis by inhibiting ADM expression and secretion.
Keywords: adenosine triphosphate metabolism; adrenomedullin; brown adipose tissue thermogenesis; ectonucleoside triphosphate diphosphohydrolase 5.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Lorenzo A. D., Romano L., Renzo L. D., Lorenzo N. D., Cenname G., Gualtieri P., Nutrition 2020, 71, 110615. - PubMed
-
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A. A., Ogurtsova K., Shaw J. E., Bright D., Williams R., IDF Diabetes Atlas Committee , Diabetes Res. Clin. Pract. 2019, 157, 107843. - PubMed
-
- Azzu V., Vacca M., Virtue S., Allison M., Vidal‐Puig A., Gastroenterology 2020, 158, 1899. - PubMed
MeSH terms
Substances
Grants and funding
- 2024ZD0523100/Noncommunicable Chronic Diseases-National Science and Technology Major Project
- 2020YFA0803800/National Key Research and Development Program of China
- 2020YFA0803803/National Key Research and Development Program of China
- 82300957/82370824/82230024/82025008/82470858/U20A20345/National Natural Science Foundation of China
- 7212123/Beijing Natural Science Foundation
LinkOut - more resources
Full Text Sources
Research Materials
