End group chemistry modulates physical properties and biomolecule release from biodegradable polyesters
- PMID: 40788135
- PMCID: PMC12338031
- DOI: 10.1039/d5tb00816f
End group chemistry modulates physical properties and biomolecule release from biodegradable polyesters
Abstract
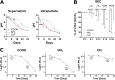
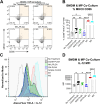
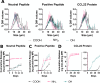
Long-acting injectable protein therapeutics are a rapidly advancing arm of pharmaceuticals. A promising and versatile class of such formulations involves encapsulation of therapeutic protein within poly(lactic-co-glycolic acid) (PLGA) degradable microparticles (MP) to shield the protein from enzymatic degradation and control the release rate. However, models based on degradation and erosion of PLGA polymer matrices do not always fully capture release behavior, due in part to electrostatic interactions between the polymer terminal group and encapsulated compound. The repertoire of functionalized PLGA polymers commercially available has now expanded to include terminal group chemistries that may significantly alter polymer characteristics including charge, hydrophobicity, and erosion. This work aims to explore how PLGA terminal group chemistry affects polymer physical properties and charged biomolecule release kinetics. PLGA with hydroxyl (PLGA-OH), amine (PLGA-NH2), or carboxylic acid (PLGA-COOH) terminal groups that have neutral, positive, or negative charge, respectively, were evaluated. Experiments assessing the physical properties of the polymers indicate PLGA-NH2 has reduced hydrophobicity, degrades faster, exhibits emulsion stabilizing behavior, and has reduced phagocytic clearance by bone marrow derived macrophages. Charged biomolecule release rates are increased from PLGA-NH2 MPs and slightly accelerated from PLGA-OH MPs, compared to PLGA-COOH MPs. These studies provide further insight into the interactions between charged biomolecules and the encapsulating polymer and could provide additional tools to tune release for various protein therapeutics that experience such interactions.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Long-acting injectable in situ forming implants: Impact of polymer attributes and API.Int J Pharm. 2025 Feb 10;670:125080. doi: 10.1016/j.ijpharm.2024.125080. Epub 2024 Dec 26. Int J Pharm. 2025. PMID: 39732214
-
Encapsulation of Therapeutic, Low-Molecular-Weight Chemokines Using a Single Emulsion, Microfluidic, Continuous Manufacturing Process.Pharmaceutics. 2025 Aug 14;17(8):1056. doi: 10.3390/pharmaceutics17081056. Pharmaceutics. 2025. PMID: 40871077 Free PMC article.
-
Internal Water-Induced Acceleration, Chemical Pathways, and Contributing Factors in the Degradation of Poly(lactic-co-glycolic acid) (PLGA) Microparticles and Devices.ACS Biomater Sci Eng. 2025 Jul 14;11(7):3932-3948. doi: 10.1021/acsbiomaterials.5c00419. Epub 2025 Jun 25. ACS Biomater Sci Eng. 2025. PMID: 40566659 Review.
-
Zinc Doped Synthetic Polymer Composites for Bone Regeneration: A Promising Strategy to Repair Bone Defects.Int J Nanomedicine. 2025 Jul 1;20:8567-8586. doi: 10.2147/IJN.S512994. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40620680 Free PMC article. Review.
References
-
- Global Markets and Manufacturing Technologies for Protein Drugs, BCC Research, 2021
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

