Milademetan in Advanced Solid Tumors with MDM2 Amplification and Wild-type TP53: Preclinical and Phase II Clinical Trial Results
- PMID: 40788172
- PMCID: PMC12521910
- DOI: 10.1158/1078-0432.CCR-25-0762
Milademetan in Advanced Solid Tumors with MDM2 Amplification and Wild-type TP53: Preclinical and Phase II Clinical Trial Results
Abstract
Purpose: Mouse double minute 2 (MDM2) is an E3 ubiquitin ligase that degrades the tumor suppressor p53. In cancers, MDM2 amplification (MDM2amp) leads to overexpression of MDM2, inducing p53 degradation and a p53-null phenotype even in the absence of TP53 mutations. We report here the preclinical and clinical activities of milademetan, a potent and selective oral small-molecule inhibitor of the MDM2-p53 interaction, in MDM2amp, TP53 wild-type (WT) solid tumors.
Patients and methods: Milademetan was tested against a variety of cell line and xenograft tumor models. This supported a phase II basket study (MANTRA-2) in patients with advanced MDM2amp, TP53-WT solid tumors. The primary endpoint was the objective response rate, and key secondary endpoints included progression-free survival and adverse events.
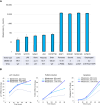
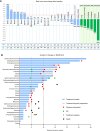
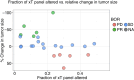
Results: Milademetan showed potent activity against MDM2amp, TP53-WT laboratory models. In the phase II trial, 40 patients received milademetan, 31 of whom had centrally confirmed molecular testing. The best overall response was 19.4% (6/31) with one confirmed response (3.2%) and five unconfirmed partial responses, including a patient with endometrial stromal sarcoma who achieved a 100% target lesion reduction. The median progression-free survival was 3.5 months (95% confidence interval, 1.8-3.7). Grade 3 or 4 adverse events observed included thrombocytopenia, neutropenia, anemia, leukopenia, and diarrhea.
Conclusion: Milademetan had a manageable safety profile and achieved responses against a variety of refractory MDM2amp, TP53-WT solid tumors, but tumor reductions were short-lived. Subsequent efforts should focus on combination strategies, further biomarker refinement, or novel MDM2 targeting approaches to achieve more durable clinical benefit.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
E.E. Dumbrava reports research or grant funding from Bayer HealthCare Pharmaceuticals Inc., Immunocore Ltd., Amgen, Aileron Therapeutics, Compugen Ltd., Gilead Sciences, Immunomedics, Bolt Biotherapeutics, Aprea Therapeutics, Bellicum Pharmaceuticals, PMV Pharma, Triumvira Immunologics, Seagen, Mereo BioPharma 5 Inc., Sanofi, Rain Oncology, Astex Pharmaceuticals, SOTIO Biotech, Poseida Therapeutics, Mersana Therapeutics, Genentech, Boehringer Ingelheim, Dragonfly Therapeutics, A2A Pharmaceuticals, Volastra, AstraZeneca, ModeX Therapeutics, Fate Therapeutics, Pfizer, and Jacobio; is on the advisory board for Bolt Biotherapeutics, Mersana Therapeutics, Orum Therapeutics, Summit Therapeutics, PMV Pharma, and Fate Therapeutics; receives speaker fees from PMV Pharma and Boehringer Ingelheim; support for travel accommodations; and expenses from American Society of Clinical Oncology, American Association for Cancer Research, LFSA Association, Rain Oncology, Banner MD Anderson Cancer Center, Triumvira Immunologics, KSMO Conference, and Boehringer Ingelheim. T.E. Stinchcombe reports personal fees from Takeda Pharmaceuticals, Gilead Sciences, Coherus Biosciences, Boehringer Ingelheim, Pfizer, AbbVie, Janssen Oncology, GSK, Genentech, Merck, and Seagen and grants from Seagen, Mirati Therapeutics, Nuvalent, Inc., and Boehringer Ingelheim outside the submitted work. M. Gounder reports grants and personal fees from Rain Oncology, Ayala Pharmaceuticals, Boehringer Ingelheim, Epizyme, Ikena Oncology, Kura Oncology, Orion Therapeutics, Foghorn Therapeutics, and Vivace Therapeutics; personal fees from AADI Bioscience, Regeneron Pharmaceuticals, and Tyme Technologies; grants from Tango Therapeutics, Erasca, GSK, and Servier during the conduct of the study; and grants in part from NIH/NCI Cancer Center Support grant P30CA008748 (to Memorial Sloan Kettering; supporting core resources). G.M. Cote reports personal fees from Gilead Sciences, C4 Therapeutics, Daiichi Sankyo, and Parabilis Medicines and other support from PharmaMar, MacroGenics, Eisai, Merck KGaA/EMD Serono Research and Development Institute, Rain Oncology, Repare Therapeutics, Foghorn Therapeutics, Jazz Pharmaceuticals, Inhibrx, Ikena Oncology, C4 Therapeutics, Bavarian Nordic, Pyxis, and Parabilis Medicines outside the submitted work; funding from Chordoma Foundation (nonprofit entity); and travel expenses from PharmaMar. T.M. Wise-Draper reports grants and personal fees from Merck & Co.; grants from Bristol Myers Squibb, Janssen Pharmaceuticals, AstraZeneca/MedImmune, and GSK; personal fees from EMD Serono, Adaptimmune, Need Inc., Caris Life Sciences, Genmab, and Replimune; and other support from High Enroll outside the submitted work. A. Forbes reports personal fees and other support from Pathos AI during the conduct of the study; personal fees and other support from Pathos AI outside the submitted work; and is an employee and equity holder in Pathos AI [Pathos AI acquired Rain Oncology in December 2023, which sponsored the clinical trial (MANTRA-2) reported in this article. A. Forbes reports that he was not involved in the conduct of the trial but contributed to analyses and interpretation of data from trial participants, and these relationships have been disclosed and are managed according to institutional and journal policies]. A. Beckmann reports personal fees from Pathos AI during the conduct of the study; personal fees from Pathos AI outside the submitted work; and is an employee and equity holder of Pathos AI [Pathos AI acquired Rain Oncology in December 2023, which sponsored the clinical trial (MANTRA-2) reported in this article. A. Beckmann reports that he was involved in the conduct of the trial but contributed to analyses and interpretation of data from trial participants, and these relationships have been disclosed and are managed according to institutional and journal policies]. E. Schadt reports being an employee and equity holder of Pathos AI [Pathos AI acquired Rain Oncology in December 2023, which sponsored the clinical trial (MANTRA-2) reported in this article. E. Schadt reports that he was not involved in the conduct of the trial but contributed to analyses and interpretation of data from trial participants, and these relationships have been disclosed and are managed according to institutional and journal policies]. N. Ku reports other support from TORL BioTherapeutics, Nurix AI, Pathos AI, Rain Oncology, and Eli Lilly and Company outside the submitted work. F. Xu reports other support from Rain Oncology during the conduct of the study. R.C. Doebele reports personal fees from Rain Oncology during the conduct of the study; personal fees from Evextabio and Boundless Bio outside the submitted work; and licensing fees for cell lines from Foundation Medicine, Casma Therapeutics, Revelio Labs, Triana Biomedicines, Pathos AI, Thermo Fisher Scientific, Roche, Personal Genome Diagnostics, Takeda Pharmaceuticals, and Loxo. C.T. Chen reports grants from Rain Oncology during the conduct of the study, as well as personal fees from Boxer Capital, Globepoint Global Advisors, Johnson & Johnson, and Mubadala Capital and grants from ADC Therapeutics, ORIC Pharmaceuticals, Takeda Pharmaceuticals, Palleon Pharmaceuticals, Pionyr Immunotherapeutics, Kinnate Biopharma, Gilead Sciences, Mersana Therapeutics, Genentech/Roche, Biohaven Pharmaceuticals, D3 Bio, Merus, Revolution Medicines, Riboscience, and Tango Therapeutics outside the submitted work. No disclosures were reported by the other authors.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

