Risk classification by pathological and biochemical prognostic factors determined by extensive exploration for metastatic hormone sensitive prostate cancer
- PMID: 40788408
- PMCID: PMC12339647
- DOI: 10.1007/s00345-025-05862-4
Risk classification by pathological and biochemical prognostic factors determined by extensive exploration for metastatic hormone sensitive prostate cancer
Abstract
Purpose: To determine prognostic parameters, we extensively examined whether physical, biochemical, and histological factors were associated with clinical outcomes in metastatic hormone sensitive prostate cancer (mHSPC) patients.
Methods: A total 822 mHSPC patients were retrospectively investigated and examined the associations between prognosis and clinicopathological parameters including BMI, initial PSA level, TNM classification, Hb, Alb, CRP, AST, ALT, LDH, ALP, Gleason grade group, and EOD score.
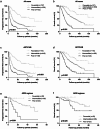
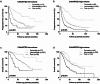
Results: According to the CHAARTED criteria, 338 (41.1%) and 484 (58.9%) patients were classified into low- and high-volume disease, respectively. In univariate and multivariate analyses, Gleason grade group, Alb, CRP, LDH, and ALP were determined as significant predictors for both PFS and OS. When mHSPC patients were classified into three group including favorable (none of risk factors), intermediate (one or two risk factors) and poor (more than three risk factors) according to these four parameters, the survival curves were significantly stratified according to the risk classification. When the risk classification was applied on the patients with low- or high-volume disease in CHAARTED criteria, worse prognosis was found in poor risk group patients with low-volume disease and favorable prognosis was found in favorable risk group patients with high-volume disease.
Conclusion: These results suggested that Gleason grade, CRP, LDH, and ALP were the independent predictors for mHSPC patients regardless of metastatic burden.
Keywords: Cancer; Epidemiology; Prostate cancer; Real-world data; mHSPC.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethical approval: This study received approval from the ethics committee of Hiroshima University (authorization number: E2021-2519) and was conducted in accordance with the Declaration of Helsinki. Informed consent: Consent was obtained by using an opt-out approach or written from all patients for the publication of this report.
Figures

Similar articles
-
Circulating Tumor Cell Count and Overall Survival in Patients With Metastatic Hormone-Sensitive Prostate Cancer.JAMA Netw Open. 2024 Oct 1;7(10):e2437871. doi: 10.1001/jamanetworkopen.2024.37871. JAMA Netw Open. 2024. PMID: 39374015 Free PMC article. Clinical Trial.
-
Comparison of survival in patients with low vs. intermediate prostate-specific antigen concentrations and development of a nomogram: a surveillance, epidemiology and end results program database study with external validation on a Chinese cohort.PeerJ. 2025 Aug 4;13:e19823. doi: 10.7717/peerj.19823. eCollection 2025. PeerJ. 2025. PMID: 40777078 Free PMC article.
-
Biopsy-detected Gleason grade 5 tumor is an additional prognostic factor in metastatic hormone-sensitive prostate cancer.J Cancer Res Clin Oncol. 2022 Mar;148(3):727-734. doi: 10.1007/s00432-021-03642-2. Epub 2021 May 4. J Cancer Res Clin Oncol. 2022. PMID: 33948720 Free PMC article.
-
Adding abiraterone to androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: A systematic review and meta-analysis.Eur J Cancer. 2017 Oct;84:88-101. doi: 10.1016/j.ejca.2017.07.003. Epub 2017 Aug 8. Eur J Cancer. 2017. PMID: 28800492 Free PMC article.
-
A systematic review of evidence on malignant spinal metastases: natural history and technologies for identifying patients at high risk of vertebral fracture and spinal cord compression.Health Technol Assess. 2013 Sep;17(42):1-274. doi: 10.3310/hta17420. Health Technol Assess. 2013. PMID: 24070110 Free PMC article.
References
-
- Siegel RL, Giaquinto AN, Jemal A (2024) Cancer statistics, 2024. CA Cancer J Clin 74(1):12–49 - PubMed
-
- Shiota M, Eto M (2016) Current status of primary pharmacotherapy and future perspectives toward upfront therapy for metastatic hormone-sensitive prostate cancer. Int J Urol 23(5):360–369 - PubMed
-
- Blas L, Shiota M, Eto M (2022) Current status and future perspective on the management of metastatic castration-sensitive prostate cancer. Cancer Treat Res Commun 32:100606 - PubMed
-
- Mohler JL, Gregory CW, Ford OH III, Kim D, Weaver CM, Petrusz P et al (2004) The androgen axis in recurrent prostate cancer. Clin Cancer Res 10(2):440–448 - PubMed
-
- Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T et al (2019) Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 17(5):479–505 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

