Efficacy of intensive antiemetic therapy including olanzapine in multiple myeloma patients treated with high-dose melphalan with autologous stem cell transplantation
- PMID: 40788411
- PMCID: PMC12339614
- DOI: 10.1007/s00520-025-09839-2
Efficacy of intensive antiemetic therapy including olanzapine in multiple myeloma patients treated with high-dose melphalan with autologous stem cell transplantation
Abstract
Purpose: Conditioning with high-dose melphalan (MEL) followed by autologous stem cell transplantation (ASCT) is the standard treatment for multiple myeloma (MM). The optimal regimen to prevent chemotherapy-induced nausea and vomiting (CINV) is unclear. We aimed to retrospectively evaluate the antiemetic effect and safety of a four-drug intensive regimen including olanzapine (OLA) on CINV in MM patients receiving MEL/ASCT.
Methods: MEL (200 mg/m2) was administered on day 1, followed by ASCT on day 3. Patients were classified into the standard group (palonosetron and dexamethasone on day 1, and aprepitant on day 1-3), and the intensive antiemetic regimen (IAR) group (palonosetron on day 1, dexamethasone on day 1-2, and aprepitant, and OLA on day 1-5). The primary endpoint was defined as no vomiting and no rescue medications (complete response) in the delayed phase (day 2-5).
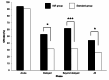
Results: There were no significant differences in baseline characteristics between the OLA (n = 68) and standard (n = 54) groups. The complete response rate in the IAR group was significantly higher in the delayed phase (52.9% vs. 31.4%, p < 0.05). Multivariate analysis revealed that the IAR was associated with the complete response rate (OR, 2.34; 95% CI, 1.09-5.00; p = 0.028). The incidence of nausea (grade 3) in the delayed phase was lower in the IAR group (44.1% vs. 75.9%, p < 0.001).
Conclusion: The four-drug intensive regimen including OLA may improve the antiemetic effect on delayed CINV while also ensuring safety in MM patients undergoing MEL/ASCT.
Keywords: Autologous stem cell transplantation multiple myeloma; Chemotherapy-induced nausea and vomiting; High-dose melphalan; Olanzapine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: This study was conducted in compliance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects (guidelines issued by the Japanese Government). This study approved by the ethics committees of the Japanese Red Cross Medical Center (reference number: 1443). Consent to participate: Information on this study was available on the webpage of the Japanese Red Cross Medical Center. Patients were notified about their participation in the study and informed that they were free to opt out. Competing Interests: The authors declare no competing interests.
Figures

Similar articles
-
Overall efficacy and safety of olanzapine 5 mg added to triplet antiemetics for an anthracycline-containing regimen in patients with breast cancer: a phase 3, double-blind, randomised, placebo-controlled trial.Lancet Oncol. 2025 Jul;26(7):960-970. doi: 10.1016/S1470-2045(25)00233-5. Epub 2025 Jun 18. Lancet Oncol. 2025. PMID: 40541214 Clinical Trial.
-
Antiemetics for adults for prevention of nausea and vomiting caused by moderately or highly emetogenic chemotherapy: a network meta-analysis.Cochrane Database Syst Rev. 2021 Nov 16;11(11):CD012775. doi: 10.1002/14651858.CD012775.pub2. Cochrane Database Syst Rev. 2021. PMID: 34784425 Free PMC article.
-
Olanzapine for the prevention and treatment of cancer-related nausea and vomiting in adults.Cochrane Database Syst Rev. 2018 Sep 21;9(9):CD012555. doi: 10.1002/14651858.CD012555.pub2. Cochrane Database Syst Rev. 2018. PMID: 30246876 Free PMC article.
-
Palonosetron, aprepitant, and dexamethasone for prevention of nausea and vomiting after high-dose melphalan in autologous transplantation for multiple myeloma: A phase II study.Int J Hematol. 2017 Apr;105(4):478-484. doi: 10.1007/s12185-016-2152-6. Epub 2016 Nov 21. Int J Hematol. 2017. PMID: 27873176 Clinical Trial.
-
A Phase II study of palonosetron, aprepitant, dexamethasone and olanzapine for the prevention of cisplatin-based chemotherapy-induced nausea and vomiting in patients with thoracic malignancy.Jpn J Clin Oncol. 2017 Sep 1;47(9):840-843. doi: 10.1093/jjco/hyx084. Jpn J Clin Oncol. 2017. PMID: 28633504 Clinical Trial.
References
-
- Cunningham D, Paz-Ares L, Milan S, Powles R, Nicolson M, Hickish T et al (1994Apr) High-dose melphalan and autologous bone marrow transplantation as consolidation in previously untreated myeloma. J Clin Oncol 12(4):759–763. 10.1200/JCO.1994.12.4.759 - PubMed
-
- Harousseau JL, Moreau P (2009) Autologous hematopoietic stem-cell transplantation for multiple myeloma. N Engl J Med 360(25):2645–2654. 10.1056/NEJMct0805626 - PubMed
-
- Berger MJ, Agarwal R, Anand S, Bagegni NA, Barbour S, Σ B, et al. NCCN Guidelines Version 2.2023 Antiemesis Continue. 2023. Available from: https://www.nccn.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

