Seralutinib for the Treatment of Pulmonary Arterial Hypertension in Adults: TORREY Open-Label Extension Study
- PMID: 40788460
- PMCID: PMC12474731
- DOI: 10.1007/s12325-025-03297-2
Seralutinib for the Treatment of Pulmonary Arterial Hypertension in Adults: TORREY Open-Label Extension Study
Abstract
Introduction: Seralutinib is an inhaled tyrosine kinase inhibitor targeting platelet-derived growth factor receptor (PDGFR) α/β, colony stimulating factor 1 receptor (CSF1R), and mast/stem cell growth factor receptor kit (c-KIT) kinases. TORREY, a phase 2, double-blind, randomized, placebo-controlled study of seralutinib in pulmonary arterial hypertension (PAH), met its primary endpoint, demonstrating a significant reduction in pulmonary vascular resistance (PVR) over placebo after 24 weeks (NCT04456998; EudraCT 2019-002669-37). We present results (as of December 5, 2024) from an open-label extension (OLE) study evaluating long-term safety, tolerability, and efficacy of seralutinib in adults with PAH (NCT04816604).
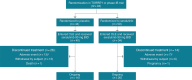
Methods: Seventy-three of 80 patients who completed TORREY (WHO Group 1 PH on stable PAH medications) and 1/8 patients from a phase 1b study (NCT03926793) enrolled in the OLE. The study design called for dosing of inhaled seralutinib 90 mg twice daily. Treatment-emergent adverse events (TEAEs) were monitored. PVR was measured after 48 weeks (week 72 from TORREY baseline). Analyses are descriptive.
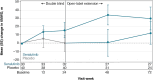
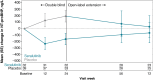
Results: At OLE entry, 34 patients continued on seralutinib (Seralutinib Continued); 40 switched from placebo to seralutinib (Placebo Crossover). Common TEAEs were headache (28.4%), coronavirus disease (COVID-19) (27.0%), and cough (23.0%). TEAEs led to seralutinib discontinuation in 20 (27.0%) patients; cough was the reason in 9/74 (12.2%). In the Seralutinib Continued group, median change in PVR from TORREY baseline to weeks 24 and 72 was - 94 dyne·s/cm5 and - 143 dyne·s/cm5, respectively (n = 28). In the Placebo Crossover group, corresponding values were - 32 dyne·s/cm5 and - 56 dyne·s/cm5, respectively (n = 27). Continued improvement in 6-min walk distance was observed. N-terminal pro-brain natriuretic peptide (NT-proBNP) significantly decreased with seralutinib during TORREY and remained stable throughout the OLE. After an increase in TORREY, NT-proBNP levels in the Placebo Crossover group nearly returned to TORREY baseline at week 72.
Conclusions: These OLE data are consistent with TORREY results and support long-term safety and efficacy of inhaled seralutinib in patients with PAH. A phase 3 study of seralutinib in PAH is underway (PROSERA, NCT05934526).
Clinical trial registration: ClinicalTrials.gov identifier NCT04816604. A Graphical Abstract is available for this article.
Keywords: Efficacy; Inhaled tyrosine kinase inhibitor; Long-term; PDGFR inhibitor; Pulmonary arterial hypertension; Pulmonary vascular resistance; Safety; Seralutinib.
Plain language summary
Pulmonary arterial hypertension is a progressive disease that causes small blood vessels in the lungs to get thicker and stiffer, making it harder for the heart to pump blood to the lungs. The phase 2 TORREY study compared inhaled seralutinib to placebo in 86 participants, showing that seralutinib reduced the pressure in blood vessels in the lungs better than placebo. This result was enhanced in participants with more severe symptoms. Seralutinib also made it easier to perform everyday activities, like walking, in patients with more symptoms. Mild-to-moderate cough was a common side effect, occurring with similar frequency in seralutinib and placebo treatment groups. In this article, we report results of an ongoing open-label extension study in which 74 patients (who participated in the TORREY study or an earlier study, collectively called the “parent studies”) received seralutinib for an extended period of time. The open-label extension looked at side effects as well as measures of improvement such as pressure in blood vessels in the lung and exercise capacity (using the 6-min walk test). Seralutinib was well tolerated. Ongoing improvements in lung blood vessel pressure and exercise capacity were observed. Because of these findings, a larger phase 3 study (PROSERA, NCT05934526) is currently underway.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Olivier Sitbon reports having received grants or contracts for research and education (payment made to the institution) from Janssen, MSD, and Ferrer; received grants or contracts for education (payment made to the institution) from AOP Orphan; payment or honoraria for lectures from AOP Orphan and Ferrer; and for educational events, lecturer, from Janssen and MSD; support for attending meetings or travel (travel costs) from MSD and Janssen; and participated on an Advisory Committee for Gossamer Bio, Inc., AOP Orphan, Enzyvant, Ferrer, Janssen, Liquidia, Roivant, MSD, and United Therapeutics. Sandeep Sahay reports having received consulting fees from Janssen, United Therapeutics, Liquidia, Keros, and Merck; support for attending meetings and/or travel from Janssen for presenting scientific work at WSPH meeting; patents submitted for Janssen as inventor, secondary titration of Uptravi; participation on an advisory board for United Therapeutics, Bayer, Liquidia, Merck, and Gossamer Bio, Inc.; participation as Chair and member of a Data Safety Monitoring Board for NIH-funded trials; holding a leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as PVD section Chair, ACCP, CHEST, Member of scientific leadership committee PHA. Pilar Escribano Subías reports having received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Janssen, MSD, Ferrer, AOT, and Aerovate; support for attending meetings and/or travel from Janssen and MSD; participation on a data safety monitoring board or advisory board for Gossamer Bio, Inc., Janssen, MSD, AOT, Aerovate, and Ferrer; and receipt of equipment, materials, drugs, medical writing, gifts, or other services from Gossamer Bio, Inc. Ronald L. Zolty reports having received consulting fees from Johnson & Johnson, United Therapeutics, Bayer, and Alnylam. John J. Ryan reports having received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from Bayer, Merck, Janssen PH, United Therapeutics, and Liquidia. Namita Sood reports having received consulting fees from United Therapeutics and Merck; membership in a Speakers Bureau for Bayer and BI; and membership in an Advisory Board for United Therapeutics and Merck. Raymond L. Benza reports having received consulting fees from Merck, United Therapeutics, KEROS, Aerovate, Insmed, and Cereno; served as an advisory committee member for Gossamer Bio, Inc.; served as an advisory board member for Altavant, Merck, Janssen, and Insmed; served on a data safety monitoring board for Janssen; and served on a scientific advisory board for Cereno. Richard N. Channick reports having received consulting fees from Gossamer Bio, Inc., Janssen, Bayer, United Therapeutics, and Third Pole; payment or honoraria for lectures from Janssen and Bayer; and participated on an advisory committee for Gossamer Bio, Inc., Janssen, and Bayer. Kelly M. Chin reports having received grants or contracts (fees to institution) for clinical studies overseen by her from Gossamer Bio, Inc., Janssen, United Therapeutics, Merck, and Altavant; received fees for work on an advisory board for Merck; received fees for work as an advisory committee member for Gossamer Bio, Inc.; received fees for work as a steering committee member for Janssen; received fees for work on an adjudication committee for United Therapeutics; and received fees for an editorial role with the American Heart Association. Robert P. Frantz reports having royalties or licenses from UpToDate; receiving consulting fees from Janssen and Liquidia; participated on a Data Safety Monitoring board for Aerovate; participated as an Advisory Committee member for Gossamer Bio, Inc.; participated on an Advisory Board for Janssen, Liquidia, Tenax Therapeutics, ShouTi, Insmed, and Merck. Hossein-Ardeschir Ghofrani reports having received consulting fees from Gossamer Bio, Inc., Aerovate, Altavant, Bayer AG, Attgeno, Janssen/Actelion, MSD/Acceleron, and Pfizer; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events as a speaker from Bayer AG, Janssen/Actelion, and MSD/Acceleron; participated as an Advisory Committee member for Aerovate, Altavant, Bayer AG, Attgeno, Janssen/Acctelion, MSD/Acceleron, and Pfizer; and as a member of a Data and Safety Monitoring Board for Insmed. Anna R. Hemnes reports having received grants or contracts from NHLBI; consulting fees from United Therapeutics, Tenax Therapeutics, Merck, and Janssen; having served as an Advisory Committee member for Gossamer Bio, Inc.; and having held stock from Tenax Therapeutics. Vallerie V. McLaughlin reports having received grant support from Aerovate, Enzyvant/Altavant, Gossamer Bio, Inc., Janssen, Merck/Acceleron, Sonovie, and Keros; consulting fees from Aerami, Aerovate, Altavant, Apollo, Bayer, CVS/Caremark, LLC, Corvista, Gossamer Bio, Inc., Janssen, Keros, Liquidia, Merck, Morphic, Regeneron, Respira, Roivant, United Therapeutics, and Vertex; and participated as an advisory committee member for Gossamer Bio, Inc. Jean-Luc Vachiéry reports having received consulting fees from Gossamer Bio, Inc., Insmed, and Tectonic; and consulting fees paid to his institution from Aerovate, Janssen, Enzyvant, Bayer HealthCare, Merck, Liquidia, and Aerami; received payment or honoraria (payment to institution) for lectures, presentations, speakers bureaus, manuscript writing or educational events from Janssen, Merck, Novartis, and Boehringer Ingelheim; received payment for expert testimony from Actelion Pharmaceuticals; received either support for attending meetings or travel (payment to institution) from Merck; participated on a data safety monitoring board (payment to institution) from Janssen, GSK, and Moderna; membership on Steering Committees for Gossamer Bio, Inc., Insmed, Merck, and United Therapeutics. Roham T. Zamanian reports having received grants or contracts for industry-supported research from United Therapeutics, Janssen, Tenax Therapeutics, and Gossamer Bio, Inc.; consulting fees from Janssen, Vivus, Morphogen-IX, Merck, and Gossamer Bio, Inc.; and stock or stock options from Selten. Luke S. Howard reports having received payment or honoraria for speaker bureau membership from Janssen; participated as an Advisory Board member for Janssen, MSD, Altavant, United Therapeutics, Keros Therapeutics, and Liquidia, and as an Advisory Committee member for Gossamer Bio, Inc.; and having stock or stock options (shareholder) at ATXA Therapeutics, iOWNA, Circular, and Calibre Biometrics. Anna ter Veer, Robert F. Roscigno, David Mottola, Ed Parsley, Richard Aranda, and Lawrence S. Zisman, report stocks or stock options from Gossamer Bio, Inc.; and employment at Gossamer Bio, Inc. Lawrence S. Zisman also reports patents related to seralutinib (assigned to Gossamer Bio, Inc., or Pulmokine) and other financial or non-financial interests (payments) from Xoma, Inc. Olivier Sitbon, Pilar Escribano Subías, Ronald L. Zolty, John F. Kingrey, John J. Ryan, Irina Sobol, Namita Sood, Raymond L. Benza, Richard N. Channick, Kelly M. Chin, Robert P. Frantz, Hossein-Ardeschir Ghofrani, Anna R. Hemnes, Vallerie V. McLaughlin, Jean-Luc Vachiéry, Roham T. Zamanian, and Luke S. Howard, report receiving medical writing and editing support, and rapid service fee support, for the present manuscript funded by Gossamer Bio, Inc. and Open Access fee support from the Université Paris-Saclay. Ethical Approval: The OLE protocol and all study-related documents were approved at each study center by the institutional review board or independent ethics committee for each site (Supplementary material). The OLE study was conducted in accordance with the tenets of the Declaration of Helsinki and the International Conference on Harmonisation-Good Clinical Practice, and all applicable laws and regulations. Prior to participation, all patients provided written informed consent.
Figures



References
-
- Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension hypertension [published correction appears in Eur Heart J. 2023 Apr 17;44(15):1312.]. Eur Heart J. 2022;43(38):3618–731. - PubMed
-
- Antoniu SA. Targeting PDGF pathway in pulmonary arterial hypertension. Expert Opin Ther Targets. 2012;16(11):1055–63. - PubMed
-
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension [published correction appears in J Am Coll Cardiol. 2014;63(7):746]. J Am Coll Cardiol. 2013;62(25 Suppl):D34-41. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

