GLP-1 Receptor Agonists and Sight-Threatening Ophthalmic Complications in Patients With Type 2 Diabetes
- PMID: 40788647
- PMCID: PMC12340654
- DOI: 10.1001/jamanetworkopen.2025.26321
GLP-1 Receptor Agonists and Sight-Threatening Ophthalmic Complications in Patients With Type 2 Diabetes
Abstract
Importance: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are associated with increased risk of diabetic retinopathy (DR) and nonarteritic anterior ischemic optic neuropathy (NAION). The risk of sight-threatening complications associated with GLP-1 RAs is underexamined.
Objective: To investigate whether the use of GLP-1 RAs in patients with T2D is associated with the development of DR, NAION, or DR complications.
Design, setting, and participants: This retrospective cohort study of adults (aged ≥18 years) with T2D and a recent hemoglobin A1c level of 6.5% or higher was conducted between January 1, 2015, and September 30, 2022, using the TriNetX database. The cohort was divided into 2 groups, adjusted for baseline characteristics through propensity score matching (PSM), based on whether the individuals received prescriptions for a GLP-1 RA. The statistical analysis was conducted on October 10, 2024.
Exposures: At least 2 prescriptions of a GLP-1 RA given 6 months apart.
Main outcomes and measures: Cox proportional hazard regression models were used to evaluate the primary outcome: association between GLP-1 RAs and the risk of incident DR, NAION, or sight-threatening complications over a 2-year follow-up period.
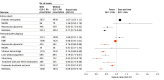
Results: After PSM, 185 066 individuals (mean [SD] age, 59.0 [12.5] years; 93 389 females [50.5%]) were prescribed GLP-1 receptor agonists. Use of GLP-1 RAs was associated with an increased incidence of DR (hazard ratio [HR], 1.07; 95% CI, 1.03-1.11), while no statistically significant difference was observed in the risk of NAION (HR, 1.26; 95% CI, 0.94-1.70). In a subgroup analysis of 32 695 patients with preexisting DR, GLP-1 RAs were not associated with progression to proliferative DR (HR, 1.06; 95% CI, 0.97-1.15) or diabetic macular edema (HR, 0.98; 95% CI, 0.95-1.01) but were associated with a lower occurrence of vitreous hemorrhages (HR, 0.74; 95% CI, 0.68-0.80), neovascular glaucoma (HR, 0.78; 95% CI, 0.68-0.88), or blindness (HR, 0.77; 95% CI, 0.73-0.82).
Conclusions and relevance: In this cohort study of individuals with T2D, GLP-1 RA use was associated with a modestly increased risk of incident DR; however, fewer patients experienced sight-threatening DR complications, including blindness, even among those with preexisting DR. These findings suggest that all patients with T2D treated with GLP-1 RAs, regardless of preexisting DR, should be regularly screened and monitored for potential complications of T2D.
Conflict of interest statement
Figures
Comment in
References
-
- Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776-785. doi: 10.1016/S2213-8587(19)30249-9 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

