Inhibition of tenascin C rescues abnormally reduced Na currents in dystrophin-deficient ventricular cardiomyocytes
- PMID: 40789177
- PMCID: PMC7618084
- DOI: 10.1152/ajpheart.00307.2025
Inhibition of tenascin C rescues abnormally reduced Na currents in dystrophin-deficient ventricular cardiomyocytes
Abstract
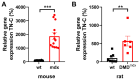
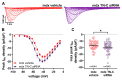
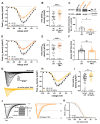
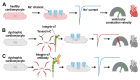
Cardiac arrhythmias significantly contribute to mortality in Duchenne muscular dystrophy (DMD), a severe muscle disease caused by dystrophin deficiency. Using the mdx mouse model for human DMD, we previously showed that the lack of dystrophin induces a significant loss of peak sodium current (INa) in ventricular cardiomyocytes. This provided a mechanistic explanation for ventricular conduction defects and concomitant arrhythmias in the dystrophic heart. The extracellular matrix protein tenascin C (TN-C), a major remodeling factor in the diseased heart, is strongly upregulated in DMD. The consequences of TN-C upregulation in the dystrophic heart, however, are unknown. Here, we tested if TN-C induces electrical remodeling in the dystrophic heart, and if inhibition of TN-C rescues peak INa loss in dystrophin-deficient ventricular cardiomyocytes. We found that cardiomyocytes from TN-C knockout (KO) mice had increased peak INa. The abnormally reduced peak INa in mdx myocytes was rescued to wild-type levels by additional TN-C KO, which was accompanied by enhanced Nav1.5 channel expression. Further, peak INa in mdx myocytes was increased by treatment of mdx mice with TN-C siRNA. Twenty-four-hour incubation of wild-type myocytes with human recombinant TN-C reduced their peak INa, an effect which could be abolished by blocking antibodies specific for the α-7 integrin subunit. Our findings suggest that TN-C induces peak INa loss in the dystrophic heart, and that inhibition of TN-C expression rescues abnormally reduced peak INa in dystrophin-deficient ventricular cardiomyocytes. TN-C inhibition emerges as a strategy to counteract ventricular conduction impairments and arrhythmias in patients with DMD.NEW & NOTEWORTHY Dystrophin deficiency in cardiomyocytes leads to abnormally reduced Na currents. These can be rescued by inhibition of the expression of tenascin C.
Keywords: Duchenne muscular dystrophy; Na current; arrhythmias; cardiomyopathy; tenascin C.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Kamdar F, Garry DJ. Dystrophin-deficient cardiomyopathy. J Am Coll Cardiol. 2016;67:2533–2546. - PubMed
-
- Perloff JK. Cardiac rhythm and conduction in Duchenne’s muscular dystrophy: a prospective study of 20 patients. J Am Coll Cardiol. 1984;3:1263–1268. - PubMed
-
- Yotsukura M, Miyagawa M, Tsuya T, Ishihara T, Ishikawa K. A 10-year follow-up study by orthogonal Frank lead ECG on patients with progressive muscular dystrophy of the Duchenne type. J Electrocardiol. 1992;25:345–353. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

