Telomere Crisis Shapes Cancer Evolution
- PMID: 40789644
- PMCID: PMC12403157
- DOI: 10.1101/cshperspect.a041688
Telomere Crisis Shapes Cancer Evolution
Abstract
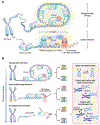
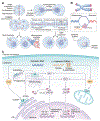
Somatic mutations arise in normal tissues and precursor lesions, often targeting cancer-driver genes involved in cell cycle regulation. Most checkpoint-mutant clones, however, remain dormant throughout an individual's lifetime and seldom progress to malignancy, implying the presence of protective mechanisms that limit their expansion and malignant transformation. One such safeguard is telomere crisis-a potent tumor-suppressive barrier that eliminates cells lacking functional checkpoints and evading p53- and pRb-mediated surveillance. While the genomic instability unleashed during telomere crisis can drive clonal evolution, cell death is typically the dominant outcome, with only a rare subset of cells escaping elimination to initiate malignancy. Recognizing the dual role of telomere crisis-suppressing tumor initiation while enabling clonal evolution-is essential for understanding early cancer development and designing strategies to eliminate tumor-initiating cells.
Copyright © 2025 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
