Dichloroacetate enhances Chemo-sensitivity in wild-type P53 breast cancer cells by modulating ABCG2 and NKG2DL
- PMID: 40789868
- PMCID: PMC12340107
- DOI: 10.1038/s41598-025-13608-w
Dichloroacetate enhances Chemo-sensitivity in wild-type P53 breast cancer cells by modulating ABCG2 and NKG2DL
Abstract
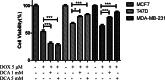
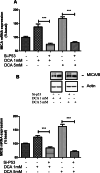
The chemotherapy drug called Adriamycin, often known as doxorubicin (Dox), is used to treat BC, including late stages. However, the emergence of resistance may reduce the efficacy of Dox in the treatment of BC. The objective of the current work is to assess how the stress ligands MICA, MICB, and ULBP1 and the expression of ABCG2 are affected by dichloroacetate (DCA). To determine the chemosensitivity of breast cancer cells, an MTT assay was performed. P-53 and ERK5 genes were silenced by Si-RNA, and gene expression level was monitored by qRT-PCR and confirmed by immunoblotting. When compared to untreated cells, our data show a significant decrease in the viability of all cell lines following a 24-hour incubation period with 5 µM of Dox in conjunction with 1 or 5 mM of DCA. The cell line MCF7 exhibited the highest rate of inhibition, followed by MDA-MB-231 and T47D. In addition, we found that DCA significantly decreases the level of ABCG2 mRNA in MCF7 cells. However, this effect was reversed in T47D and MDA-MB-231 cells. The expression of NKG2DL, MICA/B, and ULBP has also been studied. The findings indicate that DCA significantly increases the mRNA of MICA/B and ULBP1 exclusively in cells that express normal p53. ABCG2 mRNA expression is markedly upregulated in MCF7 cells upon P53 and ERK5 downregulation. Collectively, these findings showed that DCA increases the susceptibility of breast cancer cells to Dox through the upregulation of NKG2DL and the downregulation of ABCG2 expression via a p53-ERK5 dependent pathway.
Keywords: ABCG2; And chemoresistance; Breast cancer; Dichloroacetate; Doxorubicin; MICA/B; UBLP1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical statement: The author declares no conflict of interest exists.
Figures
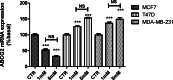



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
PARP-1 as a novel target in endocrine-resistant breast cancer.J Exp Clin Cancer Res. 2025 Jun 16;44(1):175. doi: 10.1186/s13046-025-03441-4. J Exp Clin Cancer Res. 2025. PMID: 40518539 Free PMC article.
-
11a-N-Tosyl-5-deoxi-pterocarpan, LQB-223, a novel compound with potent antineoplastic activity toward breast cancer cells with different phenotypes.J Cancer Res Clin Oncol. 2016 Oct;142(10):2119-30. doi: 10.1007/s00432-016-2212-6. Epub 2016 Aug 12. J Cancer Res Clin Oncol. 2016. PMID: 27520309 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- World Health Organization Breast Cancer. https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/ (2020).
-
- Shimura, T. et al. AKT-mediated enhanced aerobic Glycolysis causes acquired radioresistance by human tumor cells. Radiother Oncol.112, 302–307 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

