Exploring the application value of ultrasound in animal studies of stem cell therapy for intrauterine adhesions
- PMID: 40789897
- PMCID: PMC12339733
- DOI: 10.1038/s41598-025-14996-9
Exploring the application value of ultrasound in animal studies of stem cell therapy for intrauterine adhesions
Abstract
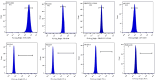
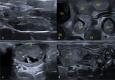
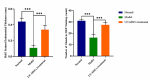
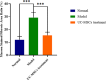
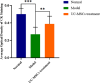
Endometrial damage leads to intrauterine adhesions (IUA), significantly impairing endometrial receptivity and fertility. Stem cell therapies, particularly umbilical cord mesenchymal stem cell (UC-MSCs), have shown promise in regenerating damaged endometrium, but prior studies have relied on ex vivo pathological evaluations. Ultrasound, as a non-invasive examination method, provides an important basis for the diagnosis, treatment and prognostic assessment of uterine adhesions in clinical practice. A series of statistical techniques were systematically used in this study, one-way ANOVA test, LSD-t test, Mann Whitney U test, Dunn's t test, and linear regression, with the aim of investigating whether ultrasound can effectively assess the effect of rat IUA model after treatment with IUA. This was an animal study involving 30 female Sprague-Dawley rats randomly divided into three groups: normal (n = 10), IUA model (n = 10), and UC-MSC therapy (n = 10). The study duration was approximately three months, including UC-MSC administration and post-treatment follow-up. IUA was induced in the model and UC-MSC groups by mechanical scraping of the uterine endometrium. Rats in the UC-MSC group received intrauterine infusions of UC-MSCs. Endometrial thickness, morphology, and continuity were evaluated pre- and post-treatment using ultrasound. Histological analysis, including H&E, Masson's staining, and CK immunohistochemistry, was performed after euthanasia. Endometrial thickness significantly increased in the UC-MSC group compared to the model group (0.34 ± 0.06 mm vs. 0.11 ± 0.03 mm, p < 0.05), while fibrosis was significantly reduced (15.11% vs. 28.14%, p < 0.05). The UC-MSC group exhibited improved endometrial morphology and glandular density compared to the model group (p < 0.05). Particularly Ultrasound findings of endometrial thickness, are significantly associated with pathological assessments (r > 0.99, p < 0.0001). This study demonstrates that ultrasound is a reliable, non-invasive tool for monitoring the therapeutic effects of UC-MSCs on endometrial regeneration in vivo.
Keywords: Cell- and Tissue-Based therapy; Endometrium; Fibrosis; Stem cell transplantation; Ultrasonography.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests. Ethics declarations: UC-MSCs and all animals studies have been approved by Ethics Committee of Yantai Yuhuangding Hospital (NO.2024-554), Date of approval: 2024.07.05 . And the title of the approved project is “ Mechanism Study on Continuous 3D-PDA Ultrasound Evaluation of Umbilical Cord Mesenchymal Stem Cells and Their Exosomes in Treating Thin Endometrium and Improving Embryo Transfer Outcome”. All animal experiments conform to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. This study did not involve human subjects.
Figures








Similar articles
-
Human amnion mesenchymal stem cells promote endometrial repair via paracrine, preferentially than transdifferentiation.Cell Commun Signal. 2024 May 31;22(1):301. doi: 10.1186/s12964-024-01656-0. Cell Commun Signal. 2024. PMID: 38822356 Free PMC article.
-
Restoration of functional endometrium in an intrauterine adhesion rat model with endometrial stromal cells transplantation.Stem Cell Res Ther. 2024 Jun 21;15(1):181. doi: 10.1186/s13287-024-03788-z. Stem Cell Res Ther. 2024. PMID: 38902788 Free PMC article.
-
Growth hormone combined with estrogen improves intrauterine adhesion fibrosis by downregulating endometrial microbial citraconic acid to target β-catenin protein.mSystems. 2025 Jul 22;10(7):e0169224. doi: 10.1128/msystems.01692-24. Epub 2025 Jun 5. mSystems. 2025. PMID: 40470939 Free PMC article.
-
Mechanistic insights into intrauterine adhesions.Semin Immunopathol. 2024 Nov 29;47(1):3. doi: 10.1007/s00281-024-01030-9. Semin Immunopathol. 2024. PMID: 39613882 Review.
-
Development of regenerative therapies targeting fibrotic endometrium in intrauterine adhesion or thin endometrium to restore uterine function.Sci China Life Sci. 2025 Aug;68(8):2264-2276. doi: 10.1007/s11427-024-2842-6. Epub 2025 Apr 11. Sci China Life Sci. 2025. PMID: 40232669 Review.
References
-
- Senturk, L. M. & Erel, C. T. Thin endometrium in assisted reproductive technology. Curr. Opin. Obstet. Gynecol.20 (3), 221–228. 10.1097/GCO.0b013e328302143c (2008). - PubMed
-
- Liu, K. E., Hartman, M. & Hartman, A. Management of thin endometrium in assisted reproduction: a clinical practice guideline from the Canadian fertility and andrology society. Reprod. Biomed. Online. 39 (1), 49–62. 10.1016/j.rbmo.2019.02.013 (2019). - PubMed
-
- Moustafa, S. M., Garneau, A. S. & Goodman, L. R. Elusive effect of endometrial Thickness: through Thick and thin. Fertil. Steril.115 (1), 89–90. 10.1016/j.fertnstert.2020.09.135 (2021). - PubMed
-
- Sarvi, F., Arabahmadi, M., Alleyassin, A., Aghahosseini, M. & Ghasemi, M. Effect of increased endometrial thickness and implantation rate by granulocyte Colony-Stimulating factor on unresponsive thin endometrium in fresh in vitro fertilization cycles: A randomized clinical trial. Obstet. Gynecol. Int.2017, 3596079. 10.1155/2017/3596079 (2017). - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

