Regeneration or Risk? A Narrative Review of BPC-157 for Musculoskeletal Healing
- PMID: 40789979
- PMCID: PMC12446177
- DOI: 10.1007/s12178-025-09990-7
Regeneration or Risk? A Narrative Review of BPC-157 for Musculoskeletal Healing
Abstract
Purpose of Review: This scoping review aims to evaluate the molecular mechanisms, therapeutic potential, and safety concerns of Body Protective Compound-157 (BPC-157) in the context of musculoskeletal healing. Given the compound’s increasing availability, popularity, and its regulatory controversies, we sought to assess the breadth and quality of preclinical and clinical data supporting its use in musculoskeletal medicine.
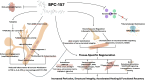
Recent Findings: BPC-157 is a synthetic pentadecapeptide originally isolated from gastric juice and has demonstrated regenerative properties across numerous animal models. It activates several overlapping pathways, notably VEGFR2 and nitric oxide synthesis via the Akt-eNOS axis, promoting angiogenesis, fibroblast activity, and neuromuscular stabilization. It also engages ERK1/2 signaling, facilitates endothelial and muscle repair, and exerts anti-inflammatory effects. These effects promote angiogenesis, fibroblast activity, and neuromuscular stabilization, particularly in poorly vascularized tissues such as tendons and myotendinous junctions. Despite broad preclinical support, human data are extremely limited. Only three pilot studies have examined BPC-157 in humans, including its use for intraarticular knee pain, interstitial cystitis, and intravenous safety/pharmacokinetics. No adverse effects were reported, but rigorous, large-scale trials are lacking.
Summary: BPC-157 demonstrates robust regenerative and cytoprotective effects in preclinical studies, positioning it as a potentially valuable tool in musculoskeletal medicine. Despite its growing popularity among athletes and its wide availability through non-regulated sources, there is minimal human data available. Until well-designed clinical trials are conducted, BPC-157 should be considered investigational, and its use approached with caution. This review highlights that given the robust preclinical evidence and high public interest, there is a critical need for well-designed human trials to assess the safety, efficacy, and clinical utility of BPC-157 in musculoskeletal medicine.
Keywords: Musculoskeletal medicine; Pentadecapeptide; Research gaps; Scoping review.
Conflict of interest statement
Declarations. Competing Interests: The authors declare no competing interests. Conflict of interest: Flynn McGuire, Riley Martinez, Annika Lenz, Lee Skinner, and Dan Cushman declare that they have no conflict of interest. Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

