Safety profile of belatacept in a real-life setting: disproportionality analysis of the WHO pharmacovigilance database
- PMID: 40790231
- PMCID: PMC12341210
- DOI: 10.1186/s40360-025-00972-6
Safety profile of belatacept in a real-life setting: disproportionality analysis of the WHO pharmacovigilance database
Abstract
Background: Belatacept is a co-stimulation blocker used in kidney transplant recipients to prevent allograft rejection. Unlike calcineurin inhibitors, belatacept is non-nephrotoxic and may carry a lower risk of cardiovascular and metabolic complications. However, the European Medicines Agency (EMA) Summary of Product Characteristics (SmPC) includes a broad range of adverse drug reactions (ADRs), mostly identified in small clinical trials with cyclosporine as a control, in patients exposed to other immunosuppressants. As real-world data on belatacept safety accumulate, a reassessment of its safety profile becomes essential.
Methods: We analyzed pharmacovigilance data from VigiBase®, the World Health Organization global safety database, to explore discrepancies between postmarketing ADR reports and the belatacept Summary of Product Characteristics (SmPC). A disproportionality analysis was performed using the Information Component, a Bayesian metric, to identify potential pharmacovigilance signals.
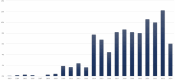
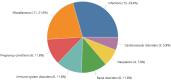
Results: We retrieved 2795 reports involving belatacept, including 424 (15.2%) fatal cases. The disproportionality analysis highlighted 51 potential signals that were not explicitly listed in the SmPC, such as Clostridium difficile infection, hepatitis B reactivation, and hemophagocytic lymphohistiocytosis. Conversely, 47 (33.1%) of the 142 ADRs classified as "common" or "very common" in the SmPC, such as Cushing's syndrome or depression, had fewer than three reports in VigiBase®.
Conclusions: This study highlights discrepancies between the belatacept SmPC and real-world pharmacovigilance data. Several labeled ADRs were not reported frequently in VigiBase®, suggesting that they may be less commonly observed in real-world settings. Others, particularly infections, warrant further scrutiny. Yet, these findings should be interpreted with caution due to the inherent limitations of pharmacovigilance data, including underreporting, reporting bias, and residual confounding. Pharmacovigilance approaches generate signals, but cannot definitively establish or exclude a causal relationship. Nonetheless, these findings suggest the need for periodic reassessment of belatacept's safety profile to ensure accurate and clinically relevant information for healthcare providers.
Keywords: Adverse drug reactions; Belatacept; Immunosuppression; Pharmacovigilance; Transplantation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: AS has been paid as a consultant by Bristol-Myers Squibb, the company that markets Nulojix®. The other authors declare no conflicts of interest.
Figures
References
-
- Grinyó JM, Del Carmen Rial M, Alberu J, Steinberg SM, Manfro RC, Nainan G, et al. Safety and efficacy outcomes 3 years after switching to Belatacept from a calcineurin inhibitor in kidney transplant recipients: results from a phase 2 randomized trial. Am J Kidney Dis Off J Natl Kidney Found. 2017;69:587–94. - PubMed
-
- Darres A, Ulloa C, Brakemeier S, Garrouste C, Bestard O, Del Bello A, et al. Conversion to Belatacept in maintenance kidney transplant patients: A retrospective multicenter European study. Transplantation. 2018;102:1545–52. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials