Heme oxygenase-1 leads to cisplatin resistance in nasopharyngeal carcinoma by reducing oxidative stress and ferroptosis
- PMID: 40790738
- PMCID: PMC12337500
- DOI: 10.1186/s12935-025-03908-6
Heme oxygenase-1 leads to cisplatin resistance in nasopharyngeal carcinoma by reducing oxidative stress and ferroptosis
Abstract
Background: Nasopharyngeal carcinoma (NPC) is a malignancy with high a mortality rate. This study investigates the impact of heme oxygenase-1 (HO-1) in cisplatin (CDDP) resistance in NPC.
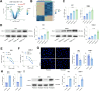
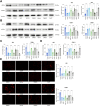
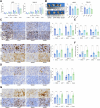
Methods: The time-dependent effect of CDDP on two NPC cell lines (HK1 and C666-1) were investigated. CDDP-resistant cells were established by exposing the parental cells to increasing concentrations of CDDP. Parental cells received treatment of the HO-1 inducer Hemin while resistant cells received treatment of the HO-1 inhibitor ZnPP to explore the influence of HO-1 activity on CDDP resistance in NPC cell lines. Erastin was used to verify the effect of ferroptosis on CDDP sensitivity in cells. Parallel settings were performed in mouse xenograft tumor models for in vivo validation.
Results: CDDP time-dependently reduced growth and mobility of NPC cells within the initial 48 h, but the cytotoxicity was no longer significantly enhanced afterward. HO-1 was upregulated in cells after CDDP treatment, showing correlation with acquired CDDP resistance. Inducing HO-1 activity in parental cells protected cells from oxidative damage, apoptosis, and ferroptosis, while suppressing HO-1 activity using ZnPP restored the therapeutic efficacy of CDDP in drug resistant cells. Moreover, the Erastin treatment also restored the cytotoxicity of CDDP to the resistant cells. In mice bearing xenograft tumors, treatment with either ZnPP or Erastin weakened the growth and weight of tumors.
Conclusion: This work suggests that HO-1 is pertinent to acquired CDDP resistance in NPC cells by suppressing oxidative stress and ferroptosis.
Keywords: CDDP resistance; Ferroptosis; HO-1; Nasopharyngeal carcinoma; Oxidative stress.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study and included experimental procedures were approved by the institutional animal care and use committee of The First Affiliated Hospital of Bengbu Medical university (approval number: 2023D136). All animal housing and experiments were conducted in strict accordance with the institutional guidelines for the care and use of laboratory animal. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Deubiquitinating enzyme UCHL1 stabilizes CAV1 to inhibit ferroptosis and enhance docetaxel resistance in nasopharyngeal carcinoma.Anticancer Drugs. 2025 Aug 1;36(7):539-548. doi: 10.1097/CAD.0000000000001721. Epub 2025 Apr 23. Anticancer Drugs. 2025. PMID: 40279201
-
CircASH1L inhibits ferroptosis and enhances cisplatin resistance by sponging miR-515-5p to regulate cell cycle-related CDCA7/RRM2 in ovarian cancer cells.Front Pharmacol. 2025 Jun 24;16:1563869. doi: 10.3389/fphar.2025.1563869. eCollection 2025. Front Pharmacol. 2025. PMID: 40630134 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
References
-
- Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. - PubMed
-
- Tang LL, Chen WQ, Xue WQ, et al. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. 2016;374(1):22–30. - PubMed
-
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. - PubMed
-
- Lee HM, Okuda KS, Gonzalez FE, Patel V. Current perspectives on nasopharyngeal carcinoma. Adv Exp Med Biol. 2019;1164:11–34. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

