Subcutaneous Abatacept in New Onset Type 1 Diabetes: Clinical and Immunological Effects
- PMID: 40790833
- PMCID: PMC12340168
- DOI: 10.1002/dmrr.70074
Subcutaneous Abatacept in New Onset Type 1 Diabetes: Clinical and Immunological Effects
Abstract
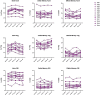
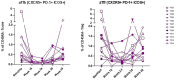
Abatacept is a CTLA4-Ig fusion protein that blocks CD80/CD86-dependent T-cell co-stimulation. When administered, Abatacept limits, to a variable degree, loss of stimulated C-peptide secretion in patients with newly-diagnosed type 1 diabetes (T1D), while reducing both circulating memory CD4+ T-cells and T follicular helper (Tfh) cells; however, its precise mechanism of action is not known. To investigate this effect, we studied 12 patients, using multi-parameter flow cytometry, who each self-administered Abatacept in subcutaneous formulation for 6 months within 100 days of diagnosis. Abatacept treatment impacted the CD4+ T cell memory compartment, inducing a reduction in T-effector cells across both conventional (Tconv) and regulatory (Treg) sub-populations. A reduction in activated Tfh cells (CXCR5+PD1+ICOS+), previously described with intravenous therapy, was replicated and extended. An integrated baseline immunological phenotype predicted Abatacept-induced preservation of C-peptide.
Keywords: Abatacept; diabetes; immunotherapy.
© 2025 The Author(s). Diabetes/Metabolism Research and Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

