Synthesis of [3H]muscimol
- PMID: 40790873
- PMCID: PMC12340338
- DOI: 10.1002/jlcr.4159
Synthesis of [3H]muscimol
Abstract
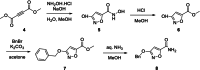
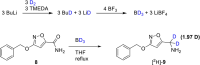
Muscimol, a potent GABAA receptor agonist and psychoactive alkaloid found in Amanita mushrooms, is widely used as a tool compound in neurochemical research. Despite its importance, synthetic access to [3H]muscimol of high specific activity has remained limited due to the challenges associated with conventional labeling strategies. Herein, we report a novel synthetic approach for the preparation of [3H]muscimol based on the reduction of a suitably protected amide precursor using in situ generated tritioborane (BT3·THF). The precursor was synthesized in four steps from dimethyl acetylenedicarboxylate, and subsequent electrophilic reduction afforded [3H]benzyl-protected muscimol in a radiochemical yield of 44 mCi (1.63 GBq) and a molar activity of 48.3 Ci/mmol (1.79 TBq/mmol). Final deprotection with HBr in acetic acid yielded [3H]muscimol·HBr in > 95% radiochemical purity. The method avoids the use of bulk tritiated water employed in established synthetic protocols and enables safe, reliable, and efficient access to this valuable radioligand for applications in GABA receptor studies.
Keywords: 3H NMR; B3H3; GABA; [3H]muscimol; tritioborane; tritium.
© 2025 The Author(s). Journal of Labelled Compounds and Radiopharmaceuticals published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
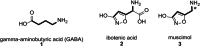
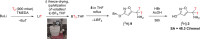

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

