Plasma tau biomarkers are distinctly associated with tau tangles and decreased with Lewy body pathology
- PMID: 40791093
- PMCID: PMC12340429
- DOI: 10.1002/alz.70562
Plasma tau biomarkers are distinctly associated with tau tangles and decreased with Lewy body pathology
Abstract
Introduction: Plasma-to-autopsy studies are essential to understand how tau blood biomarkers change in relation to Alzheimer's disease (AD) brain pathology and how they are influenced by brain co-pathologies and comorbidities.
Methods: Plasma samples from 102 brain donors of the Vallecas Alzheimer Reina Sofia cohort were analyzed using a mass spectrometry method to measure the levels of six phosphorylated and two non-phosphorylated tau biomarkers.
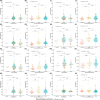
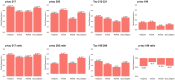
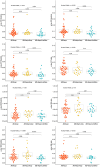
Results: In cases with high pathological burden of AD, phosphorylated tau (p-tau)217 showed associations with neurofibrillary tangle counts across all regions, while p-tau205 was primarily linked to the frontal cortex, and the non-phosphorylated tau peptides were linked to the temporal cortex. Plasma tau levels decreased as Lewy body pathology progressed, even among individuals at the same tau Braak stage. All plasma biomarkers correlated with creatinine levels, as a marker of renal dysfunction, but this effect was mitigated when using the ratios phospho/non-phospho.
Discussion: Understanding how plasma tau biomarkers are influenced by co-pathologies and comorbidities is crucial for their accurate implementation.
Highlights: The plasma phosphorylated tau (p-tau)217 ratio, followed by the p-tau205 ratio and p-tau217, were the best-performing biomarkers for detecting neuropathologically confirmed Alzheimer's disease (AD). In high AD cases, p-tau217 showed associations with neurofibrillary tangle (NFT) counts across all regions, while p-tau205 was primarily linked to the frontal cortex, and non-phosphorylated tau peptides were linked to the temporal cortex. We observed a decline in plasma tau levels as Lewy body pathology progressed, even among individuals at the same tau Braak stage. All plasma biomarkers correlated with creatinine levels, as a marker of renal dysfunction, but this effect was mitigated when using the ratios. Plasma p-tau199 displayed a distinct behavior compared to other p-tau species, showing no association with NFT counts in any brain region, but demonstrating significant correlations with brain volume and an effect on survival time.
Keywords: blood; mass spectrometry; neuropathological examination; tau phosphorylation.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Henrik Zetterberg has served on scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZpath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Quanterix, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures sponsored by Alzecure, BioArctic, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, Roche, and WebMD, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). Kaj Blennow has served as a consultant, on advisory boards, or on data monitoring committees for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Pharmatrophix, Prothena, Roche Diagnostics, and Siemens Healthineers, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this study. The other authors declare no competing interests. Author disclosures are available in the Supporting Information.
Figures

References
MeSH terms
Substances
Grants and funding
- Queen Sofia Foundation
- Familjen Rönströms Stiftelse
- #2017-00915/Swedish Research Council
- Olav Thon Foundation
- Gamla Tjänarinnor Foundation
- Erling-Persson Family Foundation
- #ADSF-21-831377-C/AD Strategic Fund and the Alzheimer's Association
- Care Research University College London Hospitals Biomedical Research Centre
- ZEN-21-848495/Alzheimer's Association 2021 Zenith Award
- #ADSF-21-831381-C/AD Strategic Fund and the Alzheimer's Association
- Swedish Dementia Foundation
- AF-968621/Alzheimerfonden
- #FO2017-0243/Hjärnfonden, Sweden
- Åhlén-stifelsen
- A2022015F/Brightfocus Foundation
- R01 AG068398/AG/NIA NIH HHS/United States
- #ADSF-24-1284328-C/AD Strategic Fund and the Alzheimer's Association
- JPND2019-466-236/European Union Joint Program for Neurodegenerative Disorders
- #RDAPB-201809-2016615/Alzheimer Drug Discovery Foundation
- UKDRI-1003/UK Dementia Research Institute at UCL
- Bluefield Project
- #ADSF-21-831376-C/AD Strategic Fund and the Alzheimer's Association
- #FO2022-0270/Hjärnfonden, Sweden
- Gun and Bertil Stohnes Foundation
- #22HLT07/NEuroBioStand
- #AF-742881/Swedish Alzheimer Foundation
- #ALFGBG-71320/Swedish State Support for Clinical Research
- Stiftelsen för Gamla Tjänarinnor
- Cure Alzheimer's Fund
- JPND2021-00694/European Union Joint Programme - Neurodegenerative Disease Research
- #1R01AG068398-01/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

