Glymphatic activity in behavioral variant frontotemporal dementia: Link with vascular function and astrocytic activation
- PMID: 40791104
- PMCID: PMC12340435
- DOI: 10.1002/alz.70561
Glymphatic activity in behavioral variant frontotemporal dementia: Link with vascular function and astrocytic activation
Abstract
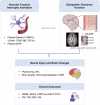
Introduction: Glymphatic activity, vascular function, and astrocytic activation play pivotal roles in the pathophysiology of frontotemporal dementia (FTD). However, their interrelationships and combined impact on clinical features remain unclear.
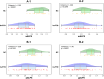
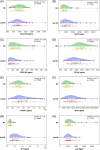
Methods: Glymphatic activity was measured by diffusion tensor image analysis along the perivascular space (DTI-ALPS) in patients with behavioral variant FTD (bvFTD, n = 61) and normal controls (NC, n = 61).
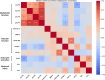
Results: Glymphatic activity in bvFTD correlated strongly with vascular dysfunction and astrocytic activation, and was associated with brain changes and clinical severity. Astrocytic activation mediates the association between vascular and glymphatic function. Astrocytic activation mediates the association between glymphatic function and clinical scales, whereas glymphatic function mediates the association between vascular function and clinical scales. The interaction effect was found between vascular function and glymphatic activity on white matter hyperintensity.
Discussion: Our findings reveal a complex interrelationship among glymphatic, vascular, and inflammatory mechanisms in FTD, highlighting their collective impact on disease severity. Highlight Vascular function, astrocytic activation, and glymphatic activity correlate with each other in frontotemporal dementia. Glymphatic activity mediates the association between vascular function and cognition. Astrocytic activation mediates the association between glymphatic function and cognition. Vascular and glymphatic function synergy drives white matter hyperintensity.
Keywords: astrocytic activation; frontotemporal dementia; glymphatic system; vascular function.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Any author disclosures are available in the Supporting Information.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

