Endonuclease Genes in Rice Are Involved in Phosphate Source Recycling by DNA Decay From Phosphate Deprivation
- PMID: 40791137
- PMCID: PMC12340663
- DOI: 10.1111/ppl.70452
Endonuclease Genes in Rice Are Involved in Phosphate Source Recycling by DNA Decay From Phosphate Deprivation
Abstract
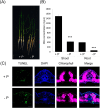
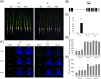
Enhancing phosphate use efficiency (PUE) has been a longstanding challenge in agriculture, as phosphate (Pi) is a crucial component of key organic molecules such as RNA, DNA, and ATP. In this study, we performed a comprehensive phylogenetic analysis of four rice and five Arabidopsis Endonuclease (ENDO) genes, which encode bifunctional nucleases capable of acting on both RNA and DNA. Our analysis revealed three distinct groups of ENDO genes: common, monocot-specific, and dicot-specific, indicating both functional conservation and diversification among monocot and dicot species. Interestingly, we found that only the monocot-specific group of rice ENDO genes exhibited differential regulation in response to P deficiency, a response not observed in Arabidopsis. Additionally, overexpression of OsPHR2, a central regulator of P homeostasis, resulted in increased DNA fragmentation and degradation, along with upregulation of OsENDO3 and OsENDO4. Transient expression assays further demonstrated that OsPHR2 activates both OsENDO3 and OsENDO4, suggesting that P-starved conditions trigger the expression of two OsPHR2-dependent rice ENDO genes. Overall, our findings suggest that OsENDO3 and OsENDO4 play a role in recycling P sources by regulating DNA decay under P deficient conditions. This insight could have significant implications for improving P use efficiency in agriculture, addressing a critical and enduring agricultural challenge.
Keywords: DNA degradation; endonuclease; phosphate recycling; phosphate starvation; rice.
© 2025 The Author(s). Physiologia Plantarum published by John Wiley & Sons Ltd on behalf of Scandinavian Plant Physiology Society.
Figures





References
-
- Aoyagi, S. , Sugiyama M., and Fukuda H.. 1998. “BEN1 and ZEN1 cDNAs Encoding S1‐Type DNases That Are Associated With Programmed Cell Death in Plants.” FEBS Letters 429, no. 2: 134–138. - PubMed
-
- Bariola, P. A. , Howard C. J., Taylor C. B., Verburg M. T., Jaglan V. D., and Green P. J.. 1994. “The Arabidopsis Ribonuclease Gene RNS1 Is Tightly Controlled in Response to Phosphate Limitation.” Plant Journal 6, no. 5: 673–685. - PubMed
-
- Canetti, L. , Lomaniec E., Elkind Y., and Lers A.. 2002. “Nuclease Activities Associated With Dark‐Induced and Natural Leaf Senescence in Parsley.” Plant Science 163, no. 4: 873–880.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

