ACSS2/AATF Drives Soluble FasL-Mediated CD8+ T Cell Apoptosis in Pancreatic Neuroendocrine Tumors
- PMID: 40791180
- PMCID: PMC12561415
- DOI: 10.1002/advs.202506883
ACSS2/AATF Drives Soluble FasL-Mediated CD8+ T Cell Apoptosis in Pancreatic Neuroendocrine Tumors
Abstract
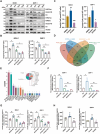
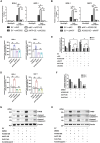
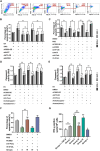
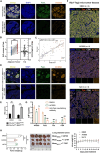
Besides the traditional carbon sources, Acetyl coenzyme A has recently been shown to be generated from acetate in various cancers, which subsequently promotes tumor growth and immune escape. However, the mechanism of Acetyl coenzyme A availability in pancreatic neuroendocrine tumors (PNETs) remains largely unknown. Herein, the metabolic-epigenetic modification driven by acetyl coenzyme A synthase 2 (ACSS2) and its effect on the Fas/FasL system in PNETs is investigated. ACSS2 is highly expressed in PNETs and significantly correlated with patient prognosis. Mechanistically, ACSS2 activity or acetate supplementation induces histone H3/H4 hyperacetylated in PNET cells. This epigenetic modification recruits the transcription factor AATF to co-regulate FasL transcription, specifically enhancing soluble FasL secretion. Secreted FasL binds Fas receptors on CD8+ T cells, activating caspase-8/3 cascades to trigger T-cell apoptosis and promote immune evasion. Notably, the finding indicated the non-redundant and synergistic effects of ACSS2 and AATF in modulating FasL expression, which might support emerging strategies for immunotherapy of PNETs.
Keywords: AATF; ACSS2; Acetyl‐CoA; Fas/FasL pathway; pancreatic neuroendocrine tumors.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Chang A., Sherman S. K., Howe J. R., Sahai V., Annu. Rev. Med. 2022, 73, 213. - PubMed
-
- Yan J., Yu S., Jia C., Li M., Chen J., Biochim. Biophys. Acta, Rev. Cancer 2020, 1874, 188367. - PubMed
-
- Strosberg J., El‐Haddad G., Wolin E., Hendifar A., Yao J., Chasen B., Mittra E., Kunz P. L., Kulke M. H., Jacene H., Bushnell D., O'Dorisio T. M., Baum R. P., Kulkarni H. R., Caplin M., Lebtahi R., Hobday T., Delpassand E., Van Cutsem E., Benson A., Srirajaskanthan R., Pavel M., Mora J., Berlin J., Grande E., Reed N., Seregni E., Öberg K., Lopera Sierra M., Santoro P., et al., N. Engl. J. Med. 2017, 376, 125. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 82173281/National Natural Science Foundation of China
- 82141129/National Natural Science Foundation of China
- 82472791/National Natural Science Foundation of China
- 82303943/National Natural Science Foundation of China
- 82403099/National Natural Science Foundation of China
- U21A20374/National Natural Science Foundation of China
- 23YF1406900/Sailing Project of Science and Technology Commission of Shanghai Municipality
- 2023M730670/China Postdoctoral Science Foundation
- 21JC1401500/Shanghai Municipal Science and Technology Major Project
- ZXXT-202201/Shanghai Municipal Health Commission's Collaborative Guidance Project for Traditional Chinese and Western Medicine
- YDZX20243100002003/Science and Technology Commission of Shanghai Municipality
- SHDC2020CR1006A/Clinical Research Plan of Shanghai Hospital Development Center
- 2021-011/Xuhui District Artificial Intelligence Medical Hospital Cooperation Project
- 25ZR1401065/Natural Science Foundation of Shanghai
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
