This is a preprint.
Cell-type-specific plasticity in synaptic, intrinsic, and sound response properties of deep-layer auditory cortical neurons after noise trauma
- PMID: 40791381
- PMCID: PMC12338564
- DOI: 10.1101/2025.07.09.663954
Cell-type-specific plasticity in synaptic, intrinsic, and sound response properties of deep-layer auditory cortical neurons after noise trauma
Update in
-
Cell type-specific plasticity in synaptic, intrinsic, and sound response properties of deep-layer cortical neurons after noise trauma.Sci Adv. 2025 Sep 19;11(38):eadx9737. doi: 10.1126/sciadv.adx9737. Epub 2025 Sep 19. Sci Adv. 2025. PMID: 40971419 Free PMC article.
Abstract
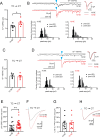
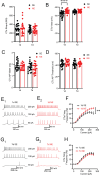
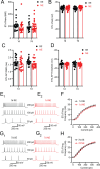
Peripheral trauma, such as noise-induced hearing loss (NIHL), triggers compensatory plasticity in the auditory cortex (ACtx) to maintain auditory function. While cortical plasticity in superficial cortical layers has been relatively well studied, the plasticity mechanisms governing deep-layer excitatory projection neurons remain less understood. Here, we investigated the plasticity of layer (L)5 extratelencephalic (ETs) and L6 corticothalamic neurons (CTs) following NIHL. Using a combination of in vitro slice electrophysiology, optogenetics, and in vivo two-photon imaging in a mouse model of NIHL, we characterized changes in evoked thalamocortical (TC) synaptic input strength, intrinsic excitability, and sound response properties. We found that TC input was initially equivalent between ETs and CTs, then shifted to CT-dominant one day after noise exposure. This shift renormalized to equivalent seven days after noise exposure and was associated with a transient increase in both the quantal size (q) in TC→CT synapses and intrinsic CT suprathreshold excitability. ETs maintained stable intrinsic properties and showed minor changes in their TC input. In vivo imaging revealed that CTs displayed a persistent elevation in sound intensity thresholds, whereas ETs transiently shifted their best frequency representation and reduced their responsiveness to high-frequency tones one day after NIHL, followed by recovery at seven days. Together, our findings highlight cell-type-specific plasticity mechanisms in deep-layer cortical neurons, enhance our understanding of cortical adaptation to peripheral damage, and highlight targets for developing therapeutic strategies to mitigate hearing loss and related disorders such as tinnitus and hyperacusis.
Keywords: auditory cortex; excitatory neurons; in vitro electrophysiology; in vivo imaging; noise-induced hearing loss; plasticity; thalamocortical circuits.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
