Combining Plant Bioactives With Antibiotics for Enhanced Antibiofilm Activity Against Uropathogenic Staphylococcus spp. and Cytotoxicity Evaluation
- PMID: 40791646
- PMCID: PMC12339163
- DOI: 10.1155/adpp/7461209
Combining Plant Bioactives With Antibiotics for Enhanced Antibiofilm Activity Against Uropathogenic Staphylococcus spp. and Cytotoxicity Evaluation
Abstract
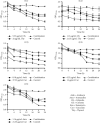
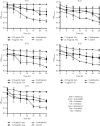
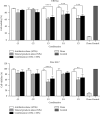
Urinary tract infections (UTIs) are one of the most important causes of morbidity and healthcare spending. Combination therapy is the treatment of choice for biofilm-associated infections due to the simultaneous action of two drugs on two separate cellular targets and their safety. This study aimed to evaluate the effect of the combination of some bioactive natural products with conventional antibiotics against the biofilm of uropathogenic Staphylococcus spp. Antibacterial and antibiofilm activities were determined by the broth microdilution test. The checkerboard method was used for combination studies. The cytotoxicity of the best synergistic combinations was evaluated on Raw 264.7 macrophage cells and urinary epithelial cells (UROtsa) using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Plumbagin also showed the best biofilm-inhibiting and eradicating activities compared to curcumin, berberine, thymol, quercetin, and gallic acid. The best synergistic combinations against biofilm inhibition and eradication were C1: cefixime (5.33 µg/mL) + thymol (32 µg/mL); C2: cefazolin (1.16 µg/mL) + thymol (21.33 µg/mL); C3: amikacin (0.18 µg/mL) + curcumin (37.33 µg/mL); C4: kanamycin (0.25 µg/mL) + curcumin (14 µg/mL); and C5: amoxicillin (1.16 µg/mL) + curcumin (21.33 µg/mL). Time-kill studies revealed that the highest antibiofilm activities of the best combinations were observed at 24 h. Eradication activities were more significant than inhibitory activities. Compared to C3, C4, and C5 combinations, C1 and C2 combinations showed less cytotoxicity against the two tested cell lines UROtsa and Raw 264.7. This study shows that the best antibiofilm synergistic effect was obtained with the combination of thymol with cefixime and cefazolin, associated with low cytotoxicity. These associations could be considered potential candidates for the development of combination therapies against Staphylococcus spp. biofilm-associated infections. While this study demonstrates promising in vitro results, further in vivo validation is necessary to confirm the efficacy and safety. Additionally, mechanistic studies are needed to understand the synergistic pathways, and future research should address scalability and formulation for clinical use.
Copyright © 2025 Ulrich Joël Tsopmene et al. Advances in Pharmacological and Pharmaceutical Sciences published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Yogo A., Yamamoto S., Sumiyoshi S., et al. Two Cases of Pyelonephritis With Bacteremia by Staphylococcus epidermidis in Male Patients With Nephrolithiasis: Case Reports and a Literature Review. Journal of Infection and Chemotherapy . 2022;28(8):1189–1192. doi: 10.1016/j.jiac.2022.04.030. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
