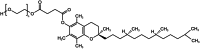Safety evaluation of d-α-tocopheryl polyethylene glycol-1000 succinate (Vitamin E TPGS) as a food additive
- PMID: 40791650
- PMCID: PMC12336668
- DOI: 10.2903/j.efsa.2025.9605
Safety evaluation of d-α-tocopheryl polyethylene glycol-1000 succinate (Vitamin E TPGS) as a food additive
Abstract
The EFSA Panel on Food Additives and Flavourings (FAF) provides a scientific opinion on the safety of d-α-tocopheryl polyethylene glycol-1000 succinate (Vitamin E TPGS) as a new food additive to be used in several food categories as emulsifier. In 2007, the EFSA AFC Panel assessed TPGS as a source of tocopherol intended to be used in foods for particular nutritional uses. The Panel considered the AFC Panel assessment relevant for the present new food additive. Compositional data showed that the proposed food additive is composed of Vitamin E TPGS monoesters (> 82% w/w of the whole preparation) and diesters (< 20% w/w of the whole preparation). Data on the hydrolysis of Vitamin E TPGS showed that the ester bond between d-α-tocopherol and succinic acid is stable under the tested conditions, as no increase in free d-α-tocopherol was observed. Vitamin E TPGS is poorly absorbed and does not represent a source of Vitamin E in the healthy population. Vitamin E TPGS does not raise a concern with respect to genotoxicity and no adverse effects on reproductive and developmental parameters were observed up to 1000 mg TPGS/kg bw per day, the highest dose tested and identified as a reference point. Due to the limitations in the available data (e.g. in reporting), the Panel decided to use an MOE approach instead of deriving an ADI. The Panel considered the calculated MOEs sufficient. Based on the available data, the Panel concluded that the use of Vitamin E TPGS as a new food additive does not raise a safety concern at the proposed use and use levels.
Keywords: Vitamin E TPGS; d‐α‐tocopheryl polyethylene glycol‐1000 succinate; emulsifier; food supplements; foods for special medical purposes.
© 2025 European Food Safety Authority. EFSA Journal published by Wiley‐VCH GmbH on behalf of European Food Safety Authority.
Figures
References
-
- Christiansen, A. , Backensfeld, T. , Kühn, S. , & Weitschies, W. (2011). Investigating the stability of the nonionic surfactants tocopheryl polyethylene glycol succinate and sucrose laurate by HPLC‐MS, DAD, and CAD. Journal of Pharmaceutical Sciences, 100(5), 1773–1782. 10.1002/jps.22408 - DOI - PubMed
-
- Commission of the European Communities . (1989a). The minimum requirements for soya‐based infant formulae and follow‐up milks. Reports of the EC Scientific Committee for Food, Twenty‐third Series. CEC.
-
- Commission of the European Communities . (1989b). Report of the Scientific Committee for Food on antioxidants. Reports of the EC Scientific Committee for Food, Twenty‐second Series. CEC.
-
- Commission of the European Communities . (1991a). First report of the Scientific Committee for Food concerning the essential requirements for weaning foods. Reports of the EC Scientific Committee for Food, Twenty‐fourth Series. CEC.
LinkOut - more resources
Full Text Sources